Method for catalyzing hybrid polymerization of alkene monomer and cyclic ester monomer by using imidazole ionic liquid
A technology of ionic liquids and polymerization methods, which is applied in the field of hybrid polymerization of imidazole ionic liquids to catalyze ethylenic monomers and cyclic ester monomers, can solve the problems of poor versatility of catalysts, harsh preparation conditions, and residual metal traces, etc., and can be designed Strong, stable, adjustable acidity, low vapor pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The molar ratio is 1:25:25:50, respectively take 1-ethyl-3-methylimidazolium chloride salt, lactide, methyl methacrylate and propylene oxide, and 0.1 g of catalyst requires 5 mL of organic solvent Take tetrahydrofuran; add the above raw materials into the reaction flask, react at 80°C for 2 hours under vacuum atmosphere, cool to room temperature, dissolve the reaction system with chloroform, and obtain the crude product; use a large amount of hydrochloric acid acidified anhydrous methanol Settling treatment, pouring the supernatant, evaporating the solvent in a fume hood, and drying in a vacuum oven at 60° C. for 10 h to obtain the corresponding hybrid copolymer.
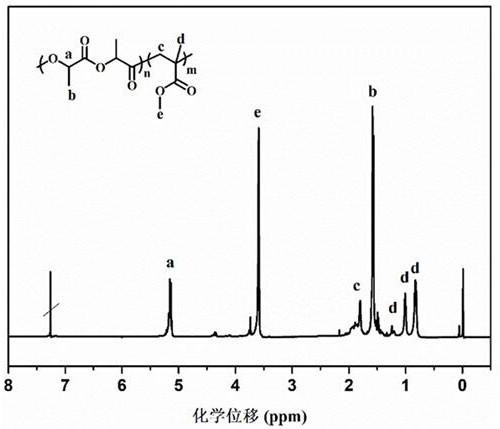
[0026] figure 1 For the proton nuclear magnetic spectrum of the poly(methyl methacrylate-co-lactide) that embodiment 1 prepares ( 1 H-NMR, CDCl 3 )picture.
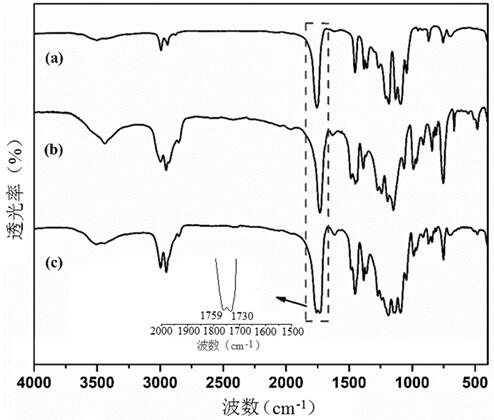
[0027] figure 2 Infrared spectrum (FTIR) comparison chart of the poly(methyl methacrylate-co-lactide) prepared in Example 1 and the respective homo...
Embodiment 2
[0030] The molar ratio is 1:50:50:100, respectively take 1-ethyl-3-methylimidazolium chloride salt, caprolactone, methyl methacrylate and propylene oxide, and 0.05 g of catalyst requires 2.5 mL of organic solvent Take tetrahydrofuran; put the above raw materials into the reaction bottle, react at 100°C for 12h under vacuum atmosphere, cool to room temperature, dissolve the reaction system with chloroform, and obtain the crude product; use a large amount of hydrochloric acid acidified anhydrous methanol Settling treatment, pouring the supernatant, evaporating the solvent in a fume hood, and drying in a vacuum oven at 60° C. for 10 h to obtain the corresponding hybrid copolymer.
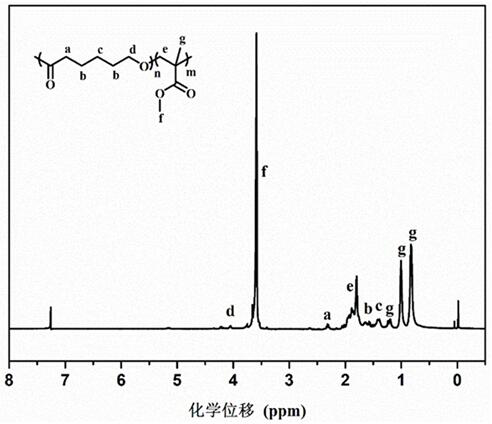
[0031] image 3 For the proton nuclear magnetic spectrum of the poly(methyl methacrylate-co-caprolactone) that embodiment 2 prepares ( 1 H-NMR, CDCl 3 )picture.
[0032] The polymer prepared in Example 2 is a copolymer of methyl methacrylate and caprolactone, rather than a mixture of both homopolyme...
Embodiment 3
[0034] The molar ratio is 1:50:50:100, respectively take 1-ethyl-3-methylimidazolium bromide, valerolactone, methyl methacrylate and propylene oxide, and 0.1 g of catalyst requires 5 mL of organic solvent Take 1,4-dioxane; put the above raw materials into the reaction flask, react at 80°C for 12h under vacuum atmosphere, cool to room temperature, dissolve the reaction system with chloroform, and obtain the crude product; acidify with a large amount of hydrochloric acid The crude product was settled with anhydrous methanol, the supernatant was poured, the solvent was evaporated in a fume hood, and dried in a vacuum oven at 60° C. for 10 h to obtain the corresponding hybrid copolymer.
[0035] Figure 4 For the proton nuclear magnetic spectrum of the poly(methyl methacrylate-co-valerolactone) prepared in embodiment 3 ( 1 H-NMR, CDCl 3 )picture.
[0036] The polymer prepared in Example 3 is a copolymer of methyl methacrylate and valerolactone, rather than a mixture of both hom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com