Compounds for treatment of neurodegenerative diseases and cancer
A technology of compounds and hydrates, applied in neurological diseases, drug combinations, organic chemistry, etc., can solve the problems of failing to improve the motor ability of PD patients, to prevent the loss of dopaminergic neurons and behavioral defects, reduce the accumulation, The effect of inhibiting the formation and accumulation of Aβ
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0218] Example 1: N-(4-(2-(dimethylamino)propyl)phenyl)-4-methyl-3-((2-(pyridin-3-yl)pyrimidin-4-yl)amino)benzene Formamide
[0219]
[0220] Step 1: Dimethyl-[1-methyl-2-(4-nitrophenyl)-ethyl]-amine]
[0221] Under nitrogen protection at room temperature, dimethylamine (1ml, 6mL) was added to a solution of 1-(4-nitrophenyl)-propan-2-one (0.7g, 4mmol) dissolved in 100mL of dichloromethane, stirred 30 min, then NaBH(OAc)3 (1.5 g) portions were added slowly over 3 hours. The reaction mixture was stirred overnight at room temperature and quenched with water. The separated aqueous layer was neutralized with 4NaOH. NaOH was added to pH = 8-9 and extracted with dichloromethane. The organic layer was dried over Na2SO4 and concentrated. The residue was slurried in MTBE and 4N HCl in dioxane (5 mL) was added. The precipitate was collected by filtration to give dimethyl-[1-methyl-2-(4-nitrophenyl)-ethyl]-amine (HCl plank) (0.8 g, 81%) as an off-white solid.
[0222] Step 2: 4-...
example 2
[0226] Example 2: 4-Methyl-N-(4-(1-methylpiperidin-4-yl)phenyl)-3-((2-(pyridin-3-yl)pyrimidin-4-yl)amino) benzamide
[0227]
[0228] 4-(1-Methylpiperidin-4-yl)-aniline (0.6 g), HATU (0.9 g) and DIPEA (1.3 mL) were added to 3-(2-pyridine-3 -yl-pyrimidin-4-ylamino)-benzoic acid (0.7g) in suspension. The reaction mixture was stirred overnight at room temperature, then poured into ice water. The slurry was stirred at room temperature, filtered, and washed with MTBE to give compound 2 (1.1 g, 81%) as a light brown solid MS: [M+H]+: 479.2; HPLC purity: 97.9%.
example 3
[0229] Example 3: Determination of Aβ40 and Aβ42 Production
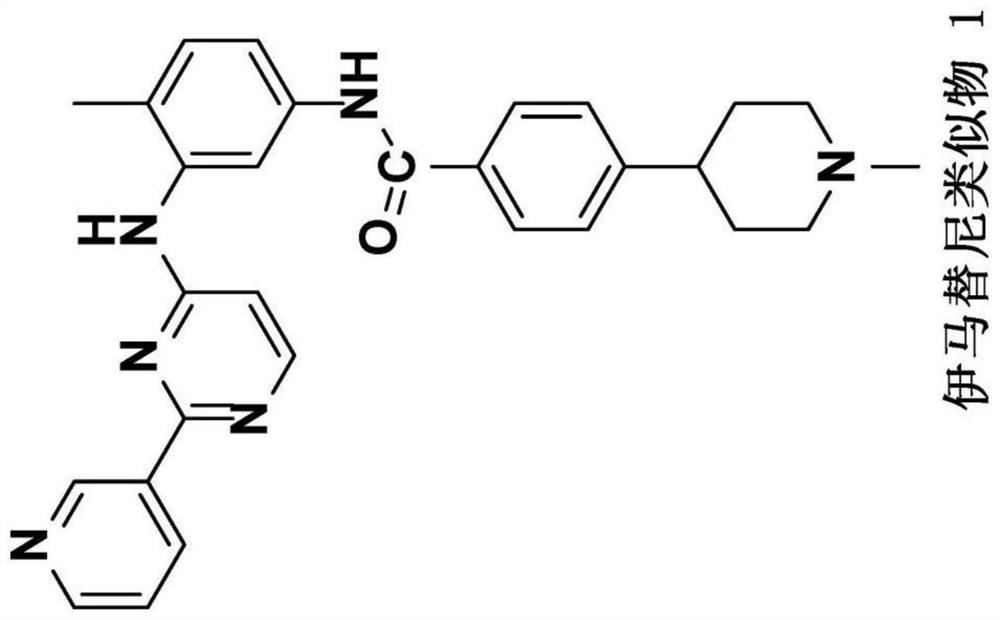
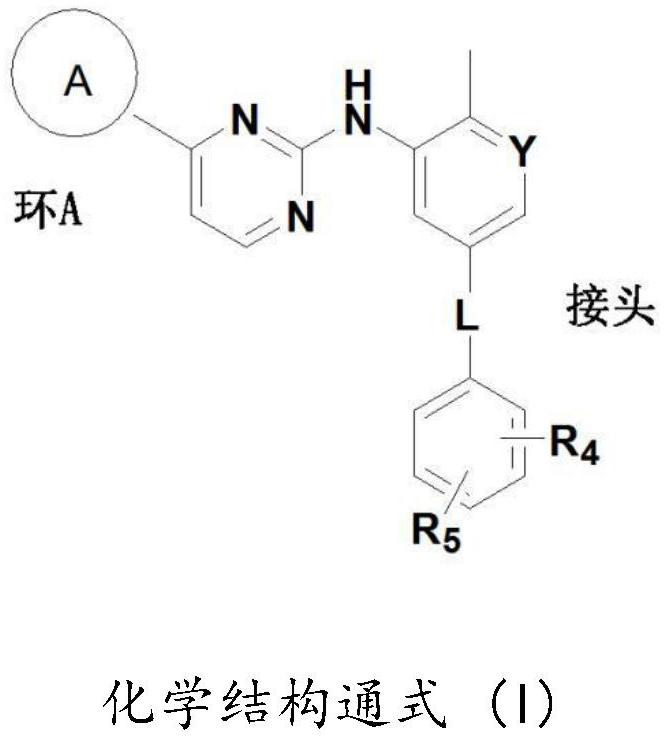
[0230] The Aβ40 and Aβ42 peptide chains are the main components that form toxic Aβ. Inhibiting the production of Aβ40 and Aβ42 and / or increasing the clearance of Aβ40 and Aβ42 is an important strategy for the development of drugs for the treatment of AD. The development of γ-secretase inhibitors, BACE inhibitors and Aβ antibodies fall into this category. The study found that the anticancer drugs imatinib and nilotinib can reduce Aβ and tau aggregation, two pathogenic features of AD. Imatinib and nilotinib decreased the levels of Aβ40 and Aβ42 by inhibiting the production of Aβ40 and Aβ42 and mediating the autophagic degradation pathway. Due to their limited brain penetration and weak activity, this patent develops and discloses more potent imatinib and nilotinib analogs with improved brain penetration. For example, imatinib analog 1 was more effective than imatinib in reducing Aβ40 and Aβ42 levels in ELISA assays...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com