Application of oligo-guluronic acid in drugs for prevention and treatment of Tau protein diseases
A technology of guluronic acid and oligomerization, applied in the field of biomedicine, can solve problems such as unsatisfactory drug efficacy, achieve prevention/treatment of tau protein disease, inhibit cell endoplasmic reticulum stress activity, and inhibit hyperphosphorylation of tau protein the effect of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of GOS and Identification of Polymerization Degree
[0050] This embodiment provides a kind of preparation method of oligomeric guluronic acid, the specific preparation method is as follows:
[0051] Sodium alginate was dissolved in 0.5M HCl, heated at 90°C for 7h and then allowed to stand overnight to separate layers. Then centrifuge at 3500rpm at low speed for 10min to obtain the precipitate for later use; use 8% NaHCO for precipitation 3 After the solution is dissolved, adjust the pH to 2.85 with HCl, let it stand for stratification, centrifuge at 3500rpm at low speed for 10 minutes, collect the precipitate, dissolve it in water, and freeze-dry to prepare PG; weigh a certain amount of PG and dissolve it in PBS, add a certain amount of alginate to crack Enzyme, react at a constant temperature at 37°C for 2 hours, then add an equal amount of enzyme solution, and continue to react at 37°C for 24 hours; after the reaction is completed, heat in a w...
Embodiment 2
[0054] Example 2. The experiment of GOS reducing the phosphorylation level of Tau protein in HEK293 / Tau cells:
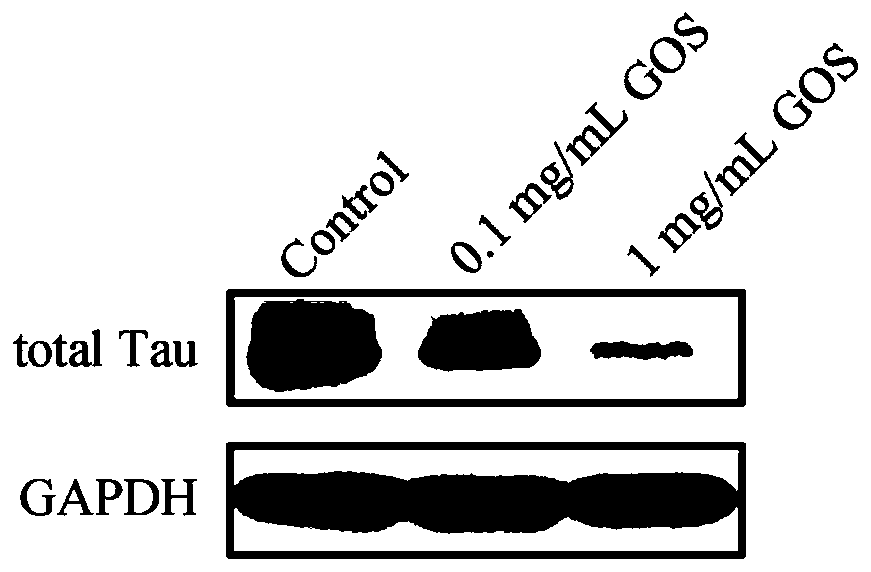
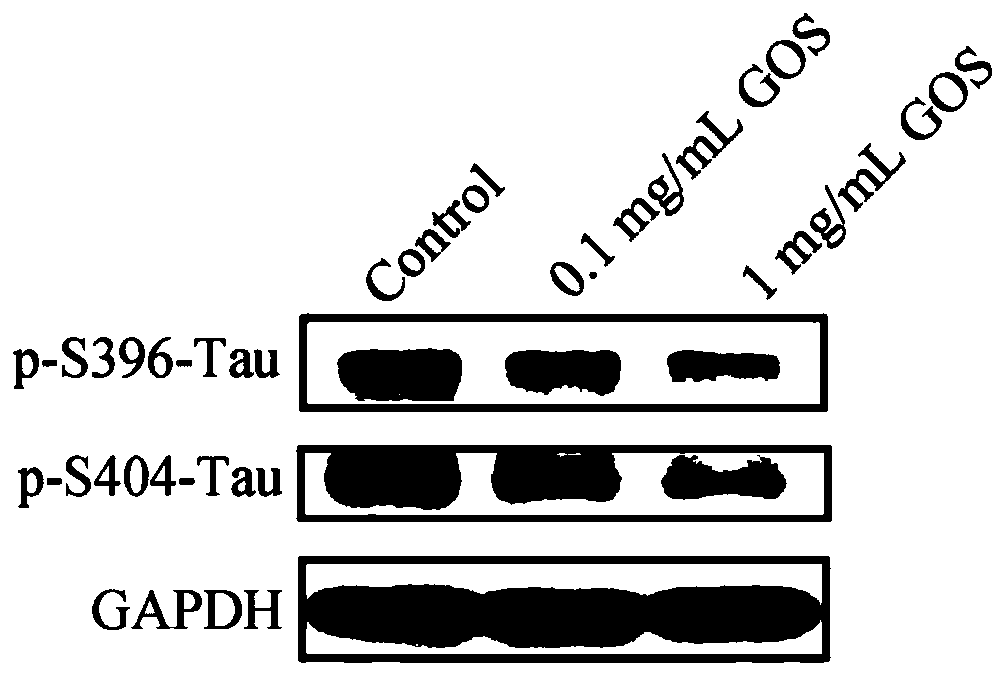
[0055] Method: HEK293 / Tau cells (2×10 6 cells / well) attached to the wall in a 6-well plate, after 4-6 hours, discard the supernatant, add 0.1mg / mL, 1mg / mL GOS to treat for 24h, use the cell lysate to extract the total protein, and then use the Western Blot method to detect the total protein The expression levels of Tau protein and phosphorylated Tau protein.
[0056] Results: The results of detecting the expression of total Tau protein were as follows: figure 1 As shown, the results of detecting the expression level of phosphorylated Tau protein are as follows figure 2 shown. Depend on figure 1It can be seen that the overexpressed Tau protein in HEK293 / Tau cells can be significantly down-regulated after GOS treatment. Depend on figure 2 It can be seen that after HEK293 / Tau cells were treated with GOS, the phosphorylation levels of Tau protein at serine 396 a...
Embodiment 3
[0057] Example 3. GOS inhibits thapsigargin-induced endoplasmic reticulum stress experiment:
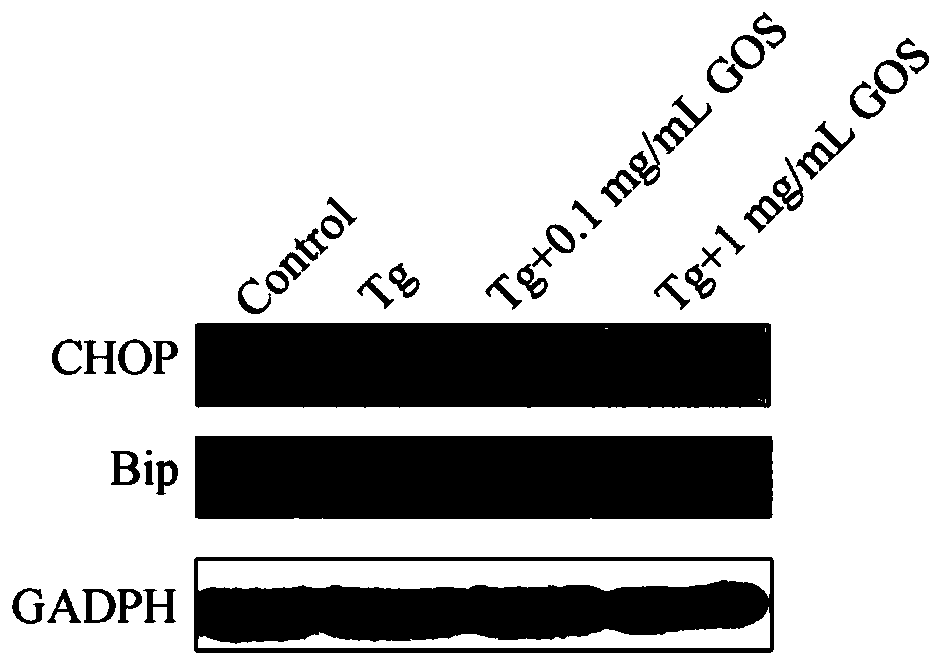
[0058] Methods: The high expression of endoplasmic reticulum chaperone Grp78 (Bip) and C / EBP homologous protein transcription factor (CHOP) is an important manifestation of endoplasmic reticulum stress. In HEK293 / Tau cells induced by thapsigargin, the effect of GOS on the expression of Bip and CHOP was detected by Western Blot. HEK293 / Tau cells (2×10 6 per well) attached to the wall in a 6-well plate, after 4-6 hours, discard the supernatant, add 0.1 mg / mL, 1 mg / mL GOS to treat for 12 hours, add 2 μg / mL thapsigargin to stimulate for 12 hours, and then extract the total protein. Using Western Blot method, it was found that the endoplasmic reticulum stress of HEK293 / Tau cells induced by GOS-treated thapsigargin was as follows: image 3 shown. Depend on image 3 It can be seen that thapsigargin can effectively induce the expression of Bip and CHOP in HEK293 / Tau cells; after GOS trea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com