Method for detecting related substances in 4-oxime methyl-1-naphthoic acid
A detection method and a technology related to substances, which are applied in measuring devices, instruments, scientific instruments, etc., can solve problems such as no patent literature reports, and achieve the effects of stable durability, good durability, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] S1: Preparation of reference substance solution: Accurately weigh 10mg of 4-oximemethyl-1-naphthoic acid reference substance in a 20mL volumetric flask, then take 1.0mL of this solution in a 50mL volumetric flask, dissolve and dilute to the mark with a solvent , mix well;
[0043] S2: Preparation of the test solution: Accurately weigh 10 mg of the 4-oximemethyl-1-naphthoic acid test product in a 20 mL volumetric flask, dissolve and dilute to the mark with a solvent, and mix well;
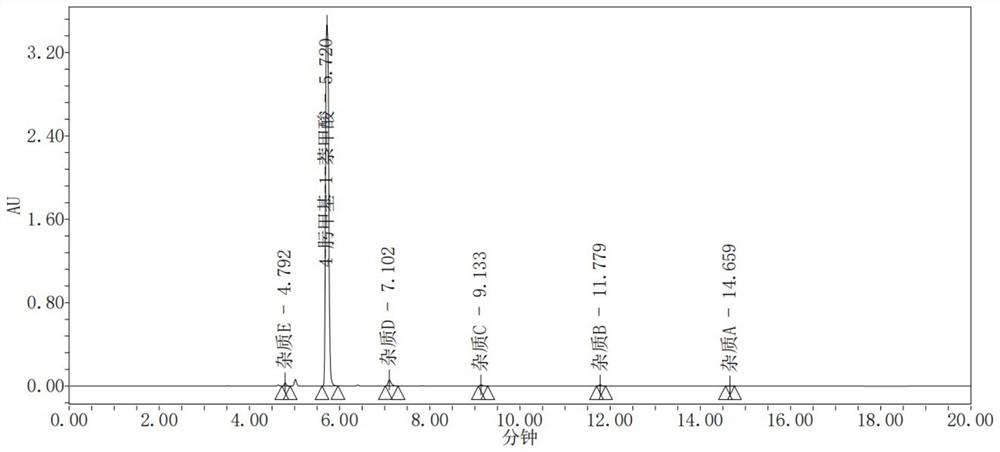
[0044] S3: HPLC measurement: Kromasil 100-5-C18 chromatographic column: 4.6mm * 100mm, 5 μ m; Mobile phase is acetonitrile: 0.01% perchloric acid aqueous solution=(30~80):(70~20)(v / v) Carry out gradient elution, the order of the gradient elution time and the volume ratio of mobile phase acetonitrile is: 0min~8min, 30%~70% operation; 8min~12min, 70%~80% operation; 12min~15min, 80% operation ;15.1min~20min, 30% operation; flow rate: 1.2mL / min; column temperature: 30°C; detection wavelength of...
Embodiment 2
[0047] The detection method of embodiment 2 and embodiment 1 is identical, and difference is the chromatographic condition of S2, specifically:
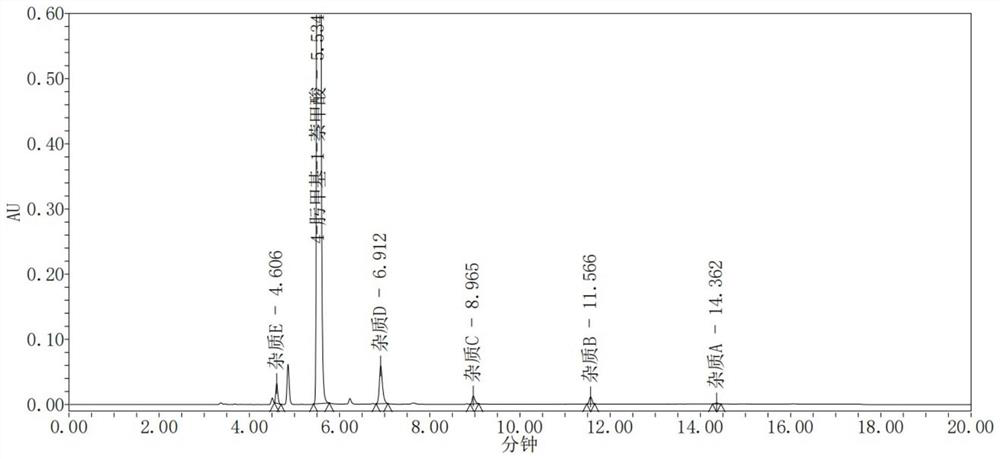
[0048] Mobile phase is acetonitrile:0.1% perchloric acid aqueous solution=(30~80):(70~20) (v / v) carries out gradient elution, and detection result is as follows figure 2 shown.
[0049] Depend on figure 2 It can be seen that each component can be effectively separated, and the content of impurity A in 4-oximemethyl-1-naphthoic acid calculated by the self-contrast method of the principal component is 0.0034%, the content of impurity B is 0.0019%, and the content of impurity C is 0.0611%. The content of impurity D is 1.1368%, the content of impurity E is 0.0486%, and the peak shape is good.
Embodiment 3
[0050] Embodiment 3: specificity test
[0051] S1: Preparation of impurity stock solution: Take impurity A, impurity B, impurity C, impurity D, and impurity E respectively, add solvent to make the concentration 0.10mg / mL, and use it as a stock solution for later use;
[0052] S2: Preparation of impurity limit solution: pipette 0.5 mL of impurity stock solution into a 20 mL volumetric flask to obtain an impurity limit solution with a concentration of 2.50 μg / mL;
[0053] S3: Preparation of system suitability solution: Accurately weigh 10 mg of 4-oximemethyl-1-naphthoic acid reference substance into a 20 mL volumetric flask, add 0.5 mL each of the above impurity stock solution, dissolve with a solvent and dilute to the mark, mix uniform. The concentration of 4-oximemethyl-1-naphthoic acid is 500 μg / mL, and the concentration of each impurity is 2.50 μg / mL;
[0054] S4: Perform measurement according to the high-performance liquid chromatography conditions in Example 1, inject ea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com