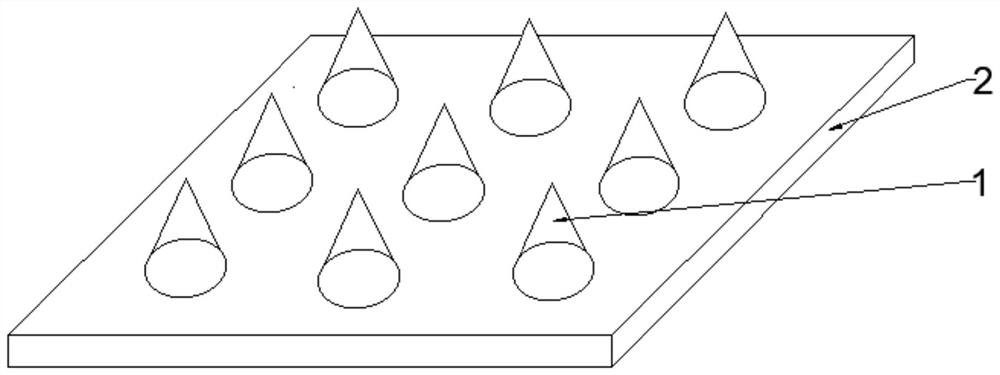
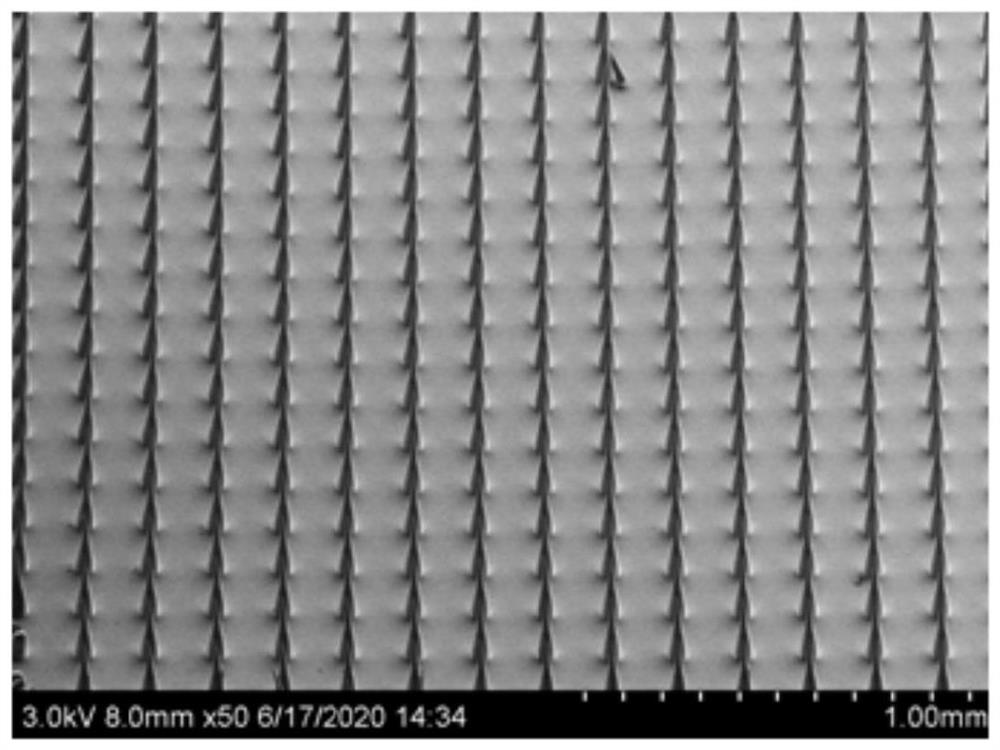
Soluble breast enlarging microneedle and preparation method thereof
A soluble, micro-needle technology, applied in the field of medical cosmetology, can solve the problems of unsafe, poor and obvious breast enhancement methods, and achieve obvious breast enhancement effects, promote breast growth, and promote the effect of chest blood circulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 0.1g polyvinylpyrrolidone, 0.9g sodium hyaluronate, dissolve in 10.0g deionized water, then weigh 0.2g N-acetyl carnosine, 0.1g acetyl hexapeptide-8, 0.05g palmitoyl tripeptide-1, 0.01g palmitoyl pentapeptide-3, 0.02g tripeptide-1, 0.01g tripeptide-1 copper, 0.05g soybean isoflavones, 0.05g hydrolyzed collagen, 0.02g kudzu root extract, 0.02g scutellaria baicalensis root extract, 0.02 Dissolve gLZ1 peptide in the above mixture to obtain microneedle body fluid; weigh 0.7g of polyvinylpyrrolidone, 0.2g of polyvinyl alcohol, 0.5g of sodium hyaluronate, and 0.1g of glycerin and dissolve them in 100g of deionized water to obtain a microneedle base fluid Inject the microneedle body liquid into the microneedle mold prepared by polydimethylsiloxane, and carry out microneedle injection molding through a vacuum injection molding process. After the injection molding is completed, remove the excess needle body liquid, and then add the microneedle base liquid to the In the mic...
Embodiment 2
[0037]Weigh 1.0g sodium hyaluronate, dissolve it in 10.0g deionized water, then weigh 0.15g acetyl hexapeptide-38, 0.1g acetyl hexapeptide-8, 0.05g palmitoyl tripeptide-1, 0.01g palm Acyl pentapeptide-3, 0.02g acetyl tetrapeptide-9, 0.01g tripeptide-10 citrulline, 0.005g stem cell exosome freeze-dried powder, 0.02g soybean isoflavones, 0.05g hydrolyzed collagen, 0.02g kudzu root Extract, 0.01g centella asiatica root extract, 0.01g houttuynia cordata extract, 0.01gLZ1 peptide were dissolved in the above mixture to obtain microneedle body fluid; weigh 0.7g of polyvinylpyrrolidone, 0.2g of polyvinyl alcohol, hyaluronic acid Dissolve 0.5 g of sodium bicarbonate and 0.1 g of glycerin in 100 g of deionized water to obtain a microneedle base fluid; inject the microneedle body fluid into a microneedle mold prepared by polydimethylsiloxane, and perform microneedle injection molding by vacuum injection molding process After the injection molding is completed, remove the excess needle bo...
Embodiment 3
[0039] Dissolve 0.7g sodium hyaluronate and 0.3g polyvinyl alcohol in 10.0g deionized water, then weigh 0.25g acetyl hexapeptide-38, 0.05g palmitoyl tripeptide-1, 0.05g tripeptide-1, 0.05g myristoyl pentapeptide-11, 0.05g myristoyl hexapeptide-4, 0.03g soybean isoflavones, 0.05g hydrolyzed collagen, 0.03g kudzu root extract, 0.01g centella asiatica root extract, 0.01g houttuynia cordata extract 0.01gLZ1 peptide was dissolved in the above mixed solution to obtain microneedle body fluid; Weighed 0.7g of polyvinylpyrrolidone, 0.2g of polyvinyl alcohol, 0.5g of sodium hyaluronate, and 0.1g of glycerin were dissolved in 100g of deionized water to obtain microneedle Needle base liquid: inject the microneedle body liquid into the microneedle mold prepared by polydimethylsiloxane, and carry out microneedle injection molding through vacuum injection molding process. After the injection molding is completed, remove the excess needle body liquid, and then inject the microneedle base Add ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| alcoholysis degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com