Method for promoting synthesis and regeneration of bimetallic catalyst by using coordination effect of chlorine
A technology of bimetallic catalysts and synthesis methods, applied in the direction of catalyst regeneration/reactivation, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc. Active site embedding and other issues, to achieve the effect of reducing application cost, good catalytic oxidation purification effect, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
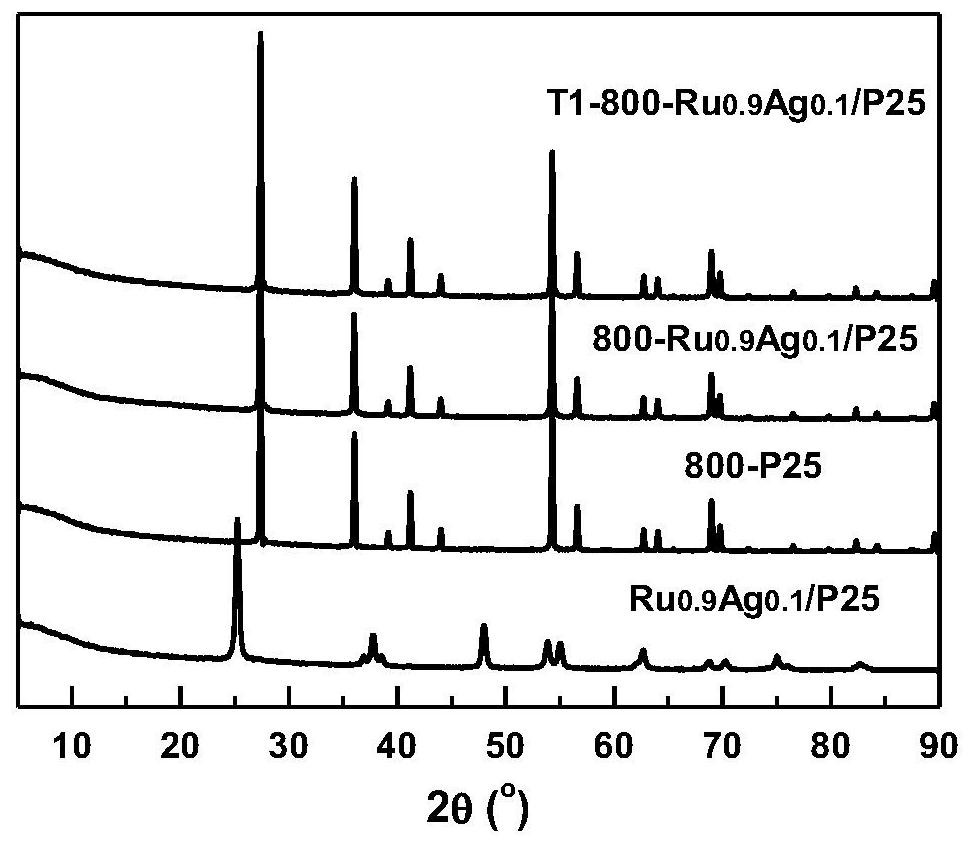
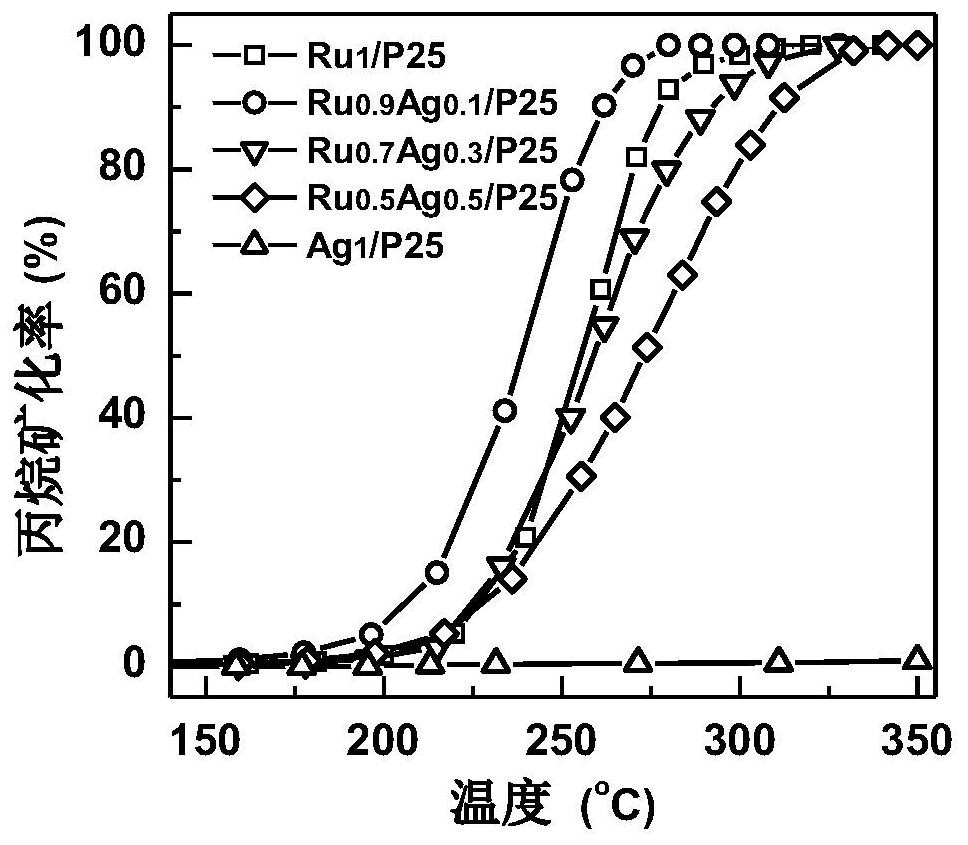
[0050] Synthetic load type single metal Ru catalyst: RUCL with 24 ml concentration of 1.25 mg (Ru) / ml 3 Aqueous solution was added in a 50 ml beaker, add 3G TiO 2 (P25) Powder, stirred rapidly for 1 h, so that the solution and the carrier are fully contacted. The solution was transferred to the heating plate, controlled the heating temperature of 80 ° C, stirring and evaporated, placed in a 100 ° C oven further dried. The resulting dark green powder was filled in the quartz tube, and the H is accessed at a gas flow rate of 50 sccm. 2 The gas is reduced, the temperature is set to 450 ° C, the time is set to 2 h, that is, a single metal Ru catalyst can be obtained, and the metal load is 1%, which is remembected as RU 1 / P25.
[0051] RUCL 3 Pre-drive solution is analyzed by UV-VIS, its analysis results figure 1 Indicated.
[0052] Take 66mg RU 1 The / p25 is placed in a quartz reaction tube, which is introduced into the reaction air containing 1% propane, and the temperature of t...
Embodiment 2
[0054] Synthetic load type single metal AG catalyst: AGNO with 24 ml concentration of 1.33 mg (Ag) / ml 3 Aqueous solution was added in a 50 ml beaker, add 3G TiO 2 (P25) Powder, stirred rapidly for 1 h, so that the solution and the carrier are fully contacted. The solution was transferred to the heating plate, controlled the heating temperature of 80 ° C, stirring and evaporated, placed in a 100 ° C oven further dried. Transfer the resulting dark green powder to the quartz tube, flows into H. 2 Restore, the temperature is set to 450 ° C, the time is set to 2 h, that is, a single metal Ru catalyst can be obtained, and the metal load is 1%, which is remembered as AG. 1 / P25.
[0055] Agno 3 Pre-drive solution is analyzed by UV-VIS, its analysis results figure 1 Indicated.
[0056] Take 66mg AG 1 The / p25 is placed in a quartz reaction tube, which is introduced into the reaction air containing 1% propane, and the temperature of the reaction is controlled at 60000 ml / (g H), and t...
Embodiment 3
[0058] Synthetic load type Ru-Ag bimetallic catalyst: put RUCL 3 Agno 3 The solution was mixed according to a certain volume, respectively, according to 21.6 + 2.4 mL, 16.8 + 7.2 mL, 12 + 12 ml of RUCL of the concentration of 1.25 mg (ru) / ml. 3 Aqueous solution and 1.33 mg (Ag) / ml AGNO 3 The aqueous solution was mixed in a 50 ml beaker, add 3 g of TiO 2 (P25) Powder, stirred rapidly for 1 h, so that the solution and the carrier are fully contacted. The solution was transferred to the heating plate, controlled the heating temperature of 80 ° C, stirring and evaporated, placed in a 100 ° C oven further dried. Transfer the resulting dark green powder to the quartz tube, flows into H. 2 Restore, the temperature is set to 450 ° C, the time is set to 2 h, that is, the Ru-Ag bimetallic catalyst can be obtained, and the total metal total load is 1%, according to the difference between the Ru and Ag ratios, respectively. 0.9 AG 0.1 / P25, RU 0.7 AG 0.3 / P25 and ru 0.5 AG 0.5 / P25.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com