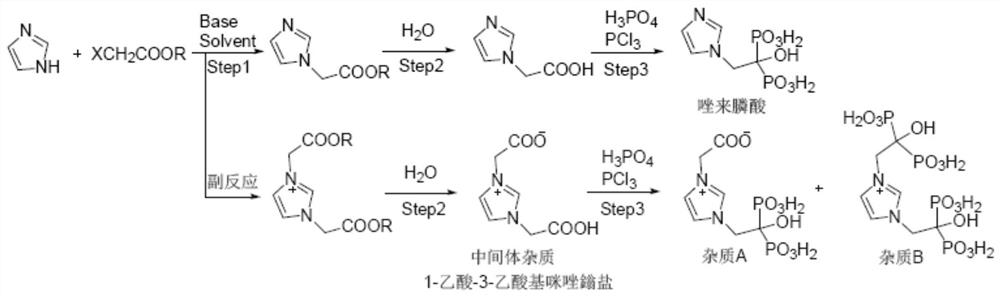
Preparation method of zoledronic acid intermediate impurity
A technology for zoledronic acid and intermediates, which is applied in the field of preparation of zoledronic acid intermediate impurity 1-acetic acid-3-acetoxyimidazolium salt, and can solve problems such as low work efficiency, unsuitability for industrialization, and poor economy , to achieve the effect of ensuring safety and effectiveness, simple and easy preparation method, ideal yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention prepares the preparation method of zoledronic acid intermediate impurity 1-acetic acid-3-acetoxy imidazolium salt comprising the following steps:
[0037] ① Nucleophilic substitution. Add imidazole and solvent into the reaction bottle, stir to dissolve, add metal iodide and alkali into the bottle, stir at 0°C-40°C for 1-3 hours; then add haloacetate into the reaction bottle, The reaction is stirred and reacted at ~40°C for 20-40 hours, and the reaction liquid is obtained after the reaction is completed, the reaction liquid is filtered, and the filtrate is concentrated.
[0038] The solvent is one or a mixture of tetrahydrofuran, 1,4-dioxane and dichloromethane.
[0039] The metal iodide is sodium iodide and / or potassium iodide. The amount of metal iodide is 0.01 to 0.1 times that of imidazole.
[0040] The alkali is one or a mixture of sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bic...
Embodiment 1)
[0048] In this embodiment, the reaction formula for preparing the zoledronic acid intermediate impurity 1-acetic acid-3-acetoxy imidazolium salt is as follows:
[0049] .
[0050] The preparation method of the zoledronic acid intermediate impurity 1-acetic acid-3-acetoxy imidazolium salt of the present embodiment comprises the following steps:
[0051] ① Nucleophilic substitution. Add 20.0g (0.29mol) of imidazole and 160mL of dichloromethane into a 500mL four-neck flask, stir and dissolve; add 60.9g (0.44mol) of potassium carbonate and 2.2g (0.015mol) of sodium iodide into the bottle, Stir at 30°C for 1±0.5 hours; then add 63.8g (0.59mol)) of methyl chloroacetate into the bottle, stir and react at 20°C to 40°C for 24±0.5 hours, stop the reaction to obtain a reaction solution. The reaction solution was filtered, and the filtrate was concentrated to 20%-40% of the original volume (25% in this example) by rotary evaporation under reduced pressure to obtain a concentrated solu...
Embodiment 2)
[0063] This embodiment prepares the preparation method of zoledronic acid intermediate impurity 1-acetic acid-3-acetoxyimidazolium salt and the rest is the same as Example 1, the difference is:
[0064] The alkali that adds in the step 1. is 0.44mol sodium hydroxide; The iodide that adds is 0.025mol potassium iodide.
[0065] After adding water to the concentrated solution in step ②, stir and react at 90°C to 100°C for 5±0.5 hours; after the reaction, pour the reaction solution into 300mL tetrahydrofuran to disperse, and filter to obtain the crude product.
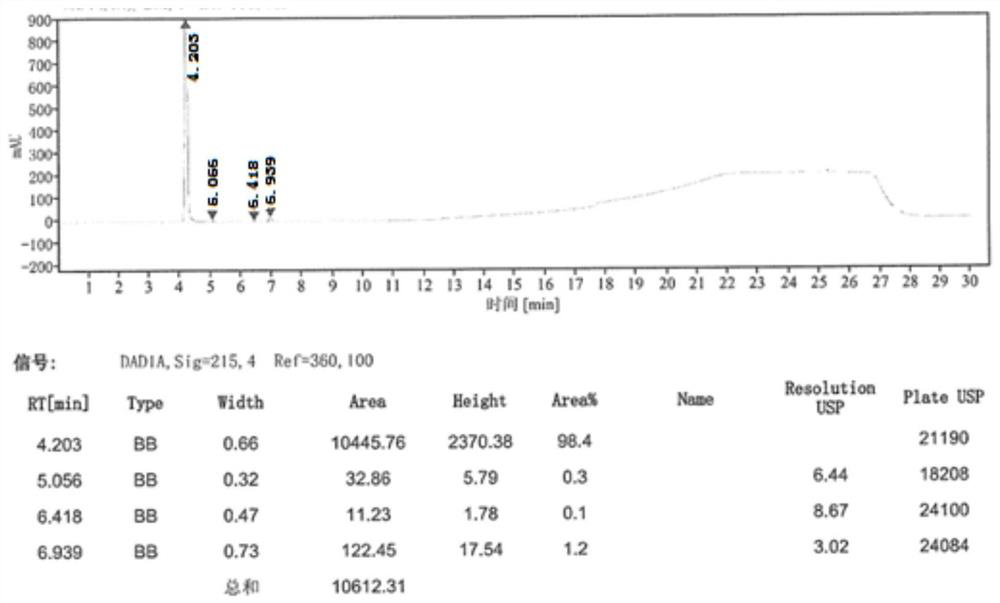
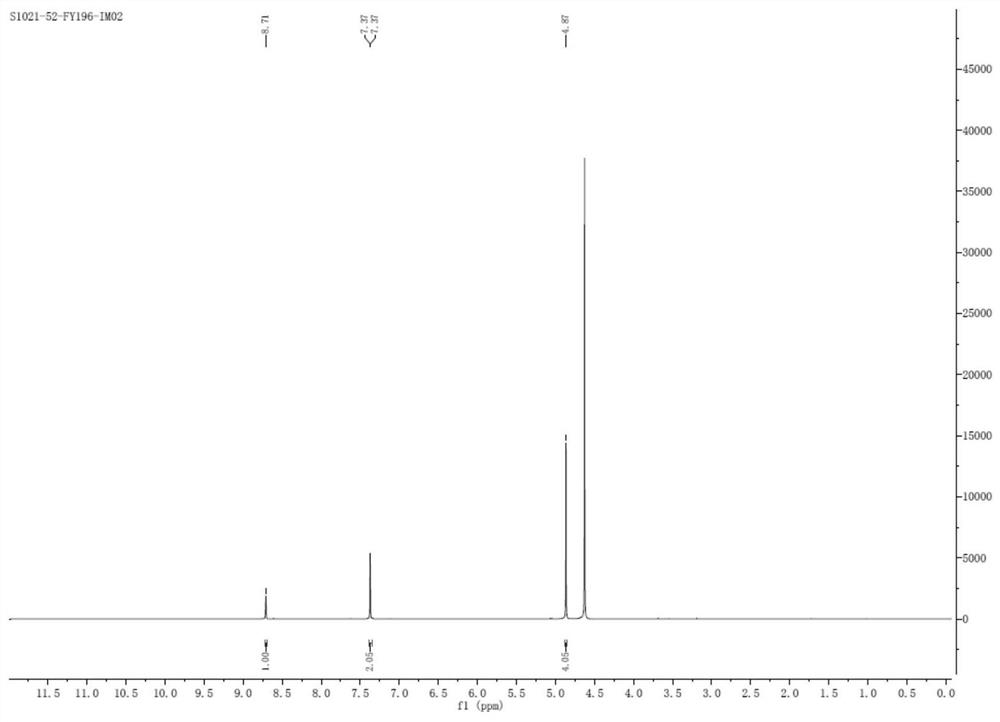
[0066] Step ③ After beating and purifying in a mixed solvent (tetrahydrofuran: water = 4:1), 30.9 g (0.17 mol) of 1-acetate-3-acetoxyimidazolium salt was obtained, with a molar yield of 57.9% and a purity of 99.2% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com