Robenidine hydrochloride, drug combination thereof and application in treatment of babesiasis
A technology of prohexidine hydrochloride and combined medication, which is applied in the field of medicine, can solve the problems of easy recurrence, treatment failure, side effects, etc., and achieve good and excellent therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The present embodiment provides a combination drug composition of clobenil hydrochloride and azithromycin for the treatment of babesiosis, and the tested drug and drug dosage are shown in Table 1.
[0082] Table 1: Combination doses and administration time of the tested drugs
[0083]
[0084] The experimental results are as follows:
[0085] (1) The EIR changes of the medication group and the control group are shown in Table 2.
[0086] Table 2: Changes in EIR of the probenazol hydrochloride + azithromycin group and the control group
[0087]
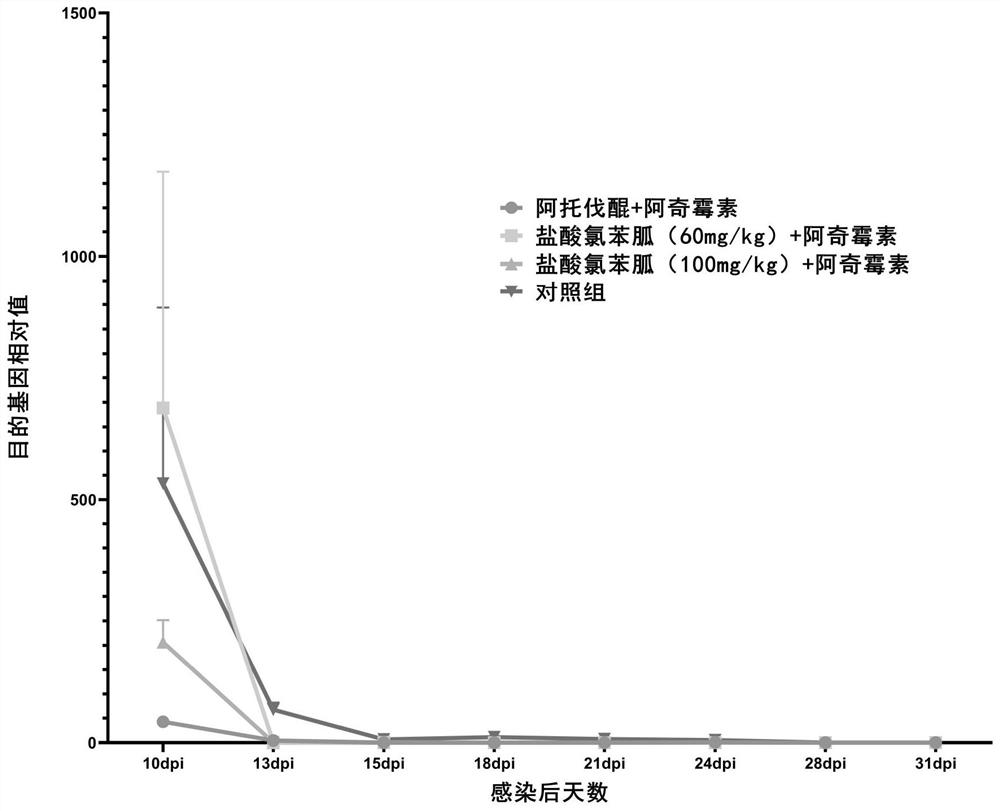
[0088] (2) qPCR results
[0089] use 2 -△△ct The relative expression of the target gene in each sample was calculated by the method, and the change trend is as follows: figure 1 shown.
[0090] (3) Immunosuppressant resurgence test results
[0091] Table 3: EIR changes of each drug group and control group in the immunosuppressant experiment
[0092]
[0093] (4) The results of the second-generation mice resurgenc...
Embodiment 2
[0098] This example provides a combination of prohexidine hydrochloride and atovaquone for the treatment of babesiosis. Table 5 shows the tested drugs and drug doses.
[0099] Table 5: Combination dosage and administration time setting of the combination of prohexenidine hydrochloride and atovaquone
[0100]
[0101] The experimental results are as follows:
[0102] (1) The EIR changes of the medication group and the control group are shown in the table below
[0103] Table 6: Changes in EIR of the chlorhexidine hydrochloride + atovaquone treatment group and the control group
[0104]
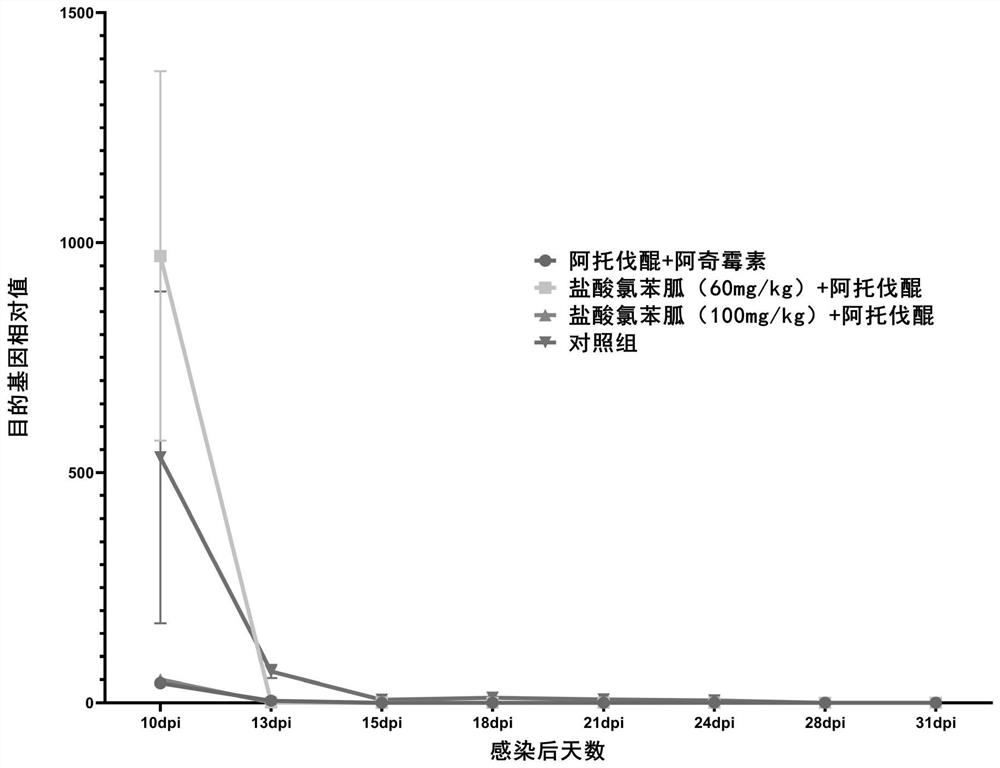
[0105] (2) qPCR results
[0106] use 2 -△△ct The relative expression of the target gene in each sample was calculated by using the method, and the change trend is as follows: figure 2 shown. (3) The results of the immunosuppressant resurgence experiment
[0107] Table 7: Changes in EIR of each medication group and control group in the immunosuppressant experiment
[0108]
[01...
Embodiment 3
[0114] This example provides a combined medicinal composition of prohexidine hydrochloride and clindamycin for the treatment of babesiosis, and the tested drugs and their doses are shown in Table 9.
[0115] Table 9: Combination dosage and administration time setting of the combination of prohexidine hydrochloride and clindamycin
[0116]
[0117] The experimental results are as follows:
[0118] (1) The EIR changes of the medication group and the control group are shown in the table below
[0119] Table 10: Changes in EIR of prohexidine hydrochloride + clindamycin treatment group and control group
[0120]
[0121]
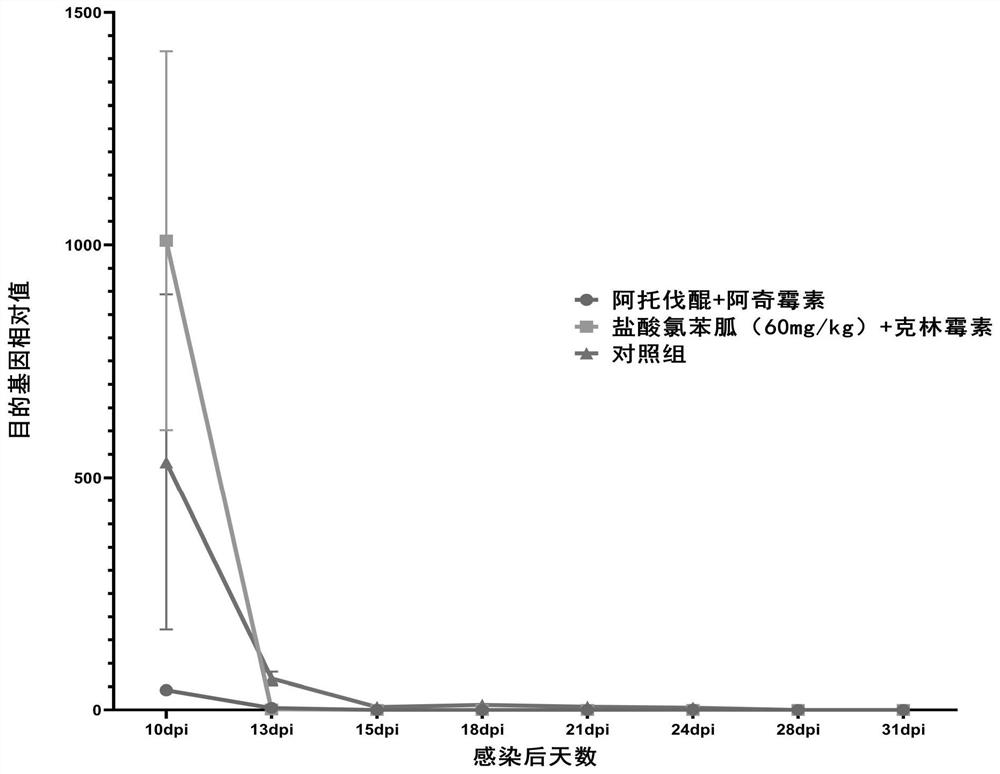
[0122] (1) qPCR results
[0123] use 2 -△△ct The relative expression of the target gene in each sample was calculated by using the method, and the change trend is as follows: image 3 shown.
[0124] (2) The results of the immunosuppressant resurgence experiment
[0125] Table 11: Changes in EIR of each medication group and control group in the imm...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap