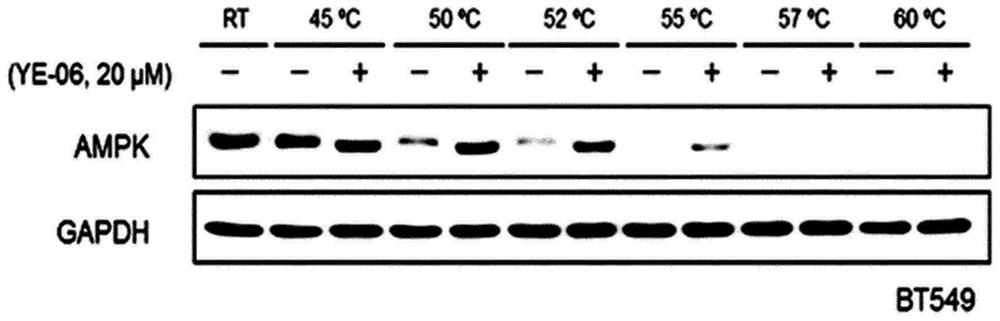
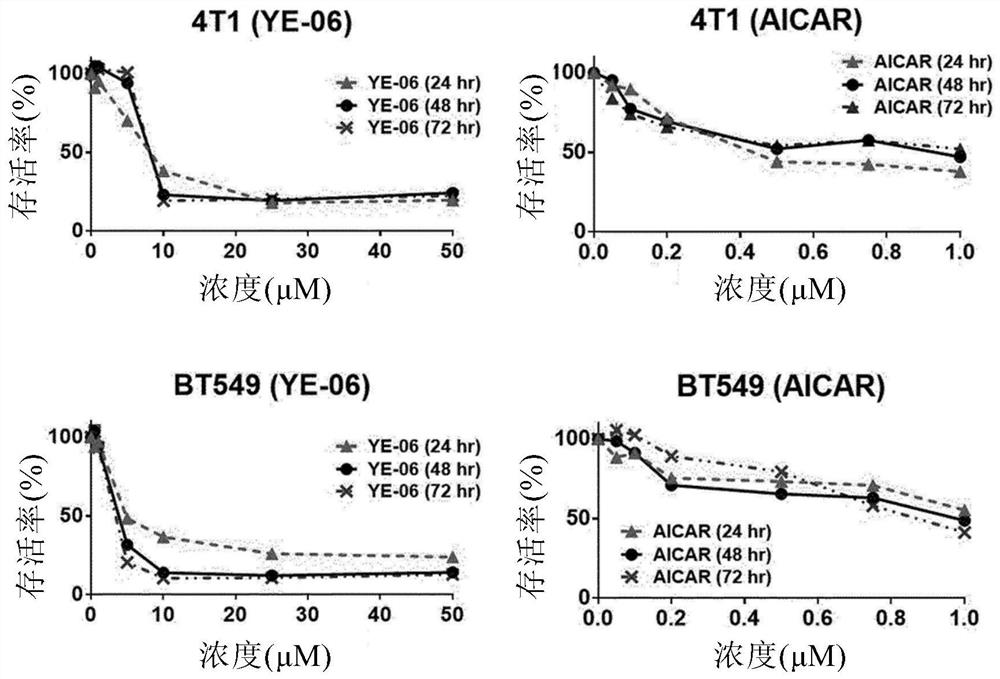
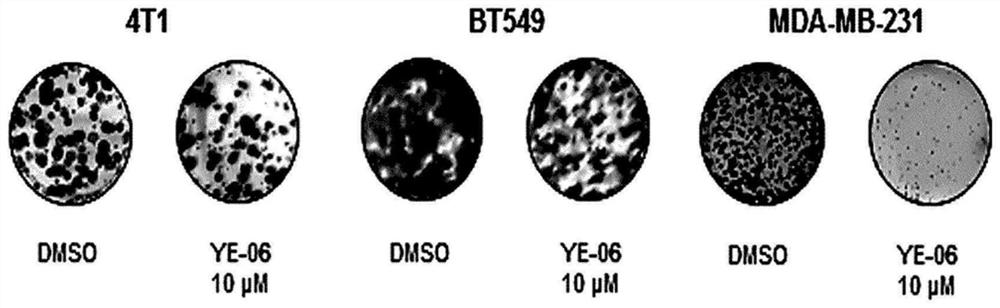
Composition for prevention, amelioration, or treatment of cancer
A technology of composition and compound, which is applied in the fields of composition, prevention, and improvement of cancer, can solve the problems of adverse reactions, low specificity of cancer cells, and low cure rate of malignant tumors, and achieve the effect of inhibiting cancer metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1-15
[0156] [Preparation Example 1-15] Synthesis of Candidate Compounds
[0157] Compounds of Preparation Examples 1-15 shown in Table 1 below were prepared using Method 1 or Method 2.
[0158] [Table 1]
[0159]
[0160]
[0161] [method 1]
[0162] The method comprises the steps of: adding an acetophenone derivative (1 equivalent), a benzaldehyde derivative (1 equivalent) and NaOH (1 equivalent) to an ethanol solvent, and then stirring at room temperature; after the reaction is completed, adding water to the reaction mixture , and then extracted with ethyl acetate; and collected the organic solvent layer, the collected material was washed once with water, washed with anhydrous MgSO 4 The washed material was dried, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel chromatography.
[0163] [Method 2]
[0164] The method comprises the steps of: adding acetophenone derivatives (1 equivalent), 4-(tetrahydro-2H-pyran-2-...
Embodiment 1
[0165] [Example 1] (E)-1-(4-aminophenyl)-3-(2,4-dimethoxyphenyl)prop-2-en-1-one (YE-01)
[0166] Starting from 4-aminoacetophenone (0.30 g, 2.22 mmol), 2,4-dimethoxybenzaldehyde (0.37 g, 2.22 mmol) and NaOH (0.09 g, 2.22 mmol) according to method 1 above. The residue was purified by silica gel chromatography (developing solvent: ethyl acetate / n-hexane=1:2→1:1) to obtain the compound of Preparation Example 1 (0.15 g, yield 23.0%) as a yellow solid. R f 0.33 (ethyl acetate / n-hexane=1:1); 1 H-NMR (400MHz, CDCl 3 )δ3.85(s,3H),3.89(s,3H),6.47(d,J=2.0Hz,1H),6.52(dd,J=8.4,2.4Hz,1H),6.69(d,J=8.4 Hz, 2H), 7.55(d, J=15.6Hz, 1H), 7.56(d, J=8.8Hz, 1H), 7.92(d, J=8.4Hz, 2H), 8.02(d, J=15.6Hz, 1H); 13 C-NMR (100MHz, CDCl 3 ) 55.6, 55.7, 98.7, 105.5, 114.1, 117.8, 120.7, 129.4, 130.8, 131.1, 139.0, 150.9, 160.4, 162.8, 189.1 ppm.
Embodiment 2
[0167] [Example 2] (E)-1-(4-aminophenyl)-3-(2,5-dimethoxyphenyl)prop-2-en-1-one (YE-02)
[0168] Starting from 4-aminoacetophenone (0.50 g, 3.70 mmol), 2,5-dimethoxybenzaldehyde (0.62 g, 3.70 mmol) and NaOH (0.15 g, 3.70 mmol) according to method 1 above. The residue was purified by silica gel chromatography (developing solvent: ethyl acetate / n-hexane=1:2→1:1) to obtain the compound of Preparation Example 2 (0.66 g, yield 62.5%) as a yellow solid. R f 0.36 (ethyl acetate / n-hexane=1:1); 1 H-NMR (400MHz, CDCl 3 )δ3.81(s,3H),3.85(s,3H),4.19(br s,2H),6.69(d,J=8.8Hz,2H),6.86(d,J=8.8Hz,1H),6.91 (dd, J=8.8,2.8Hz,1H),7.16(d,J=2.8Hz,1H),7.58(d,J=15.6Hz,1H),7.92(d,J=8.8Hz,2H),8.04 (d,J=15.6Hz,1H); 13 C-NMR (100MHz, CDCl 3 ) 56.0, 56.3, 112.7, 113.9, 114.1, 116.8, 123.3, 125.2, 128.9, 131.3, 138.6, 151.2, 153.4, 153.7, 188.8ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com