Application of 4-(o-ascorbic acid)-(+)-catechin and detection method of 2,3-trans leucocyanidin
An ascorbic acid and detection method technology, applied in organic chemical methods, measuring devices, instruments, etc., can solve the problems of reduced detection speed, affected accuracy, and difficulty in distinguishing, and achieves the analysis of carbon flow distribution and improves reliability and accuracy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Screening of 2,3-trans leucocyanidin candidate endogenous indicators in plants
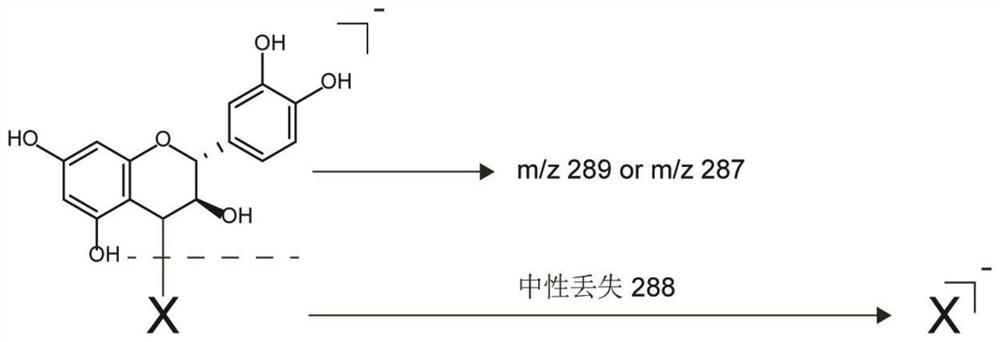
[0066] The design idea of the endogenous indicator screening experiment in this example is that because 2,3-trans leucoanthocyanidin can spontaneously transform into an electrophilic (+)-catechin-type carbocation in plants , which can react with nucleophiles to form relatively stable adducts. Therefore, the content of 2,3-trans leucocyanidin can be obtained by detecting the content of the adduct, and the relationship between the two content is positively correlated.
[0067] As shown in formula (1), according to the molecular structure of 2,3-trans leucocyanidin, its C4 position is easy to form (+)-catechin or (-)-epicatechin type carbocation ; this position is attacked by a potential nucleophile, and finally a stable covalent bond is formed; therefore, the general structural formula of the adduct formed by the reaction of 2,3-trans leucocyanidin with a nucleophile is the formu...
Embodiment 2
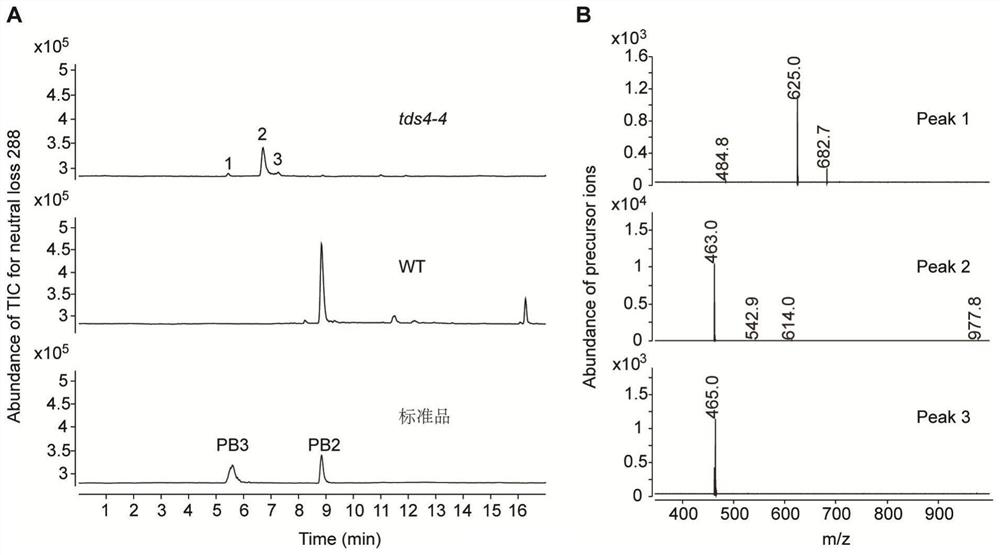
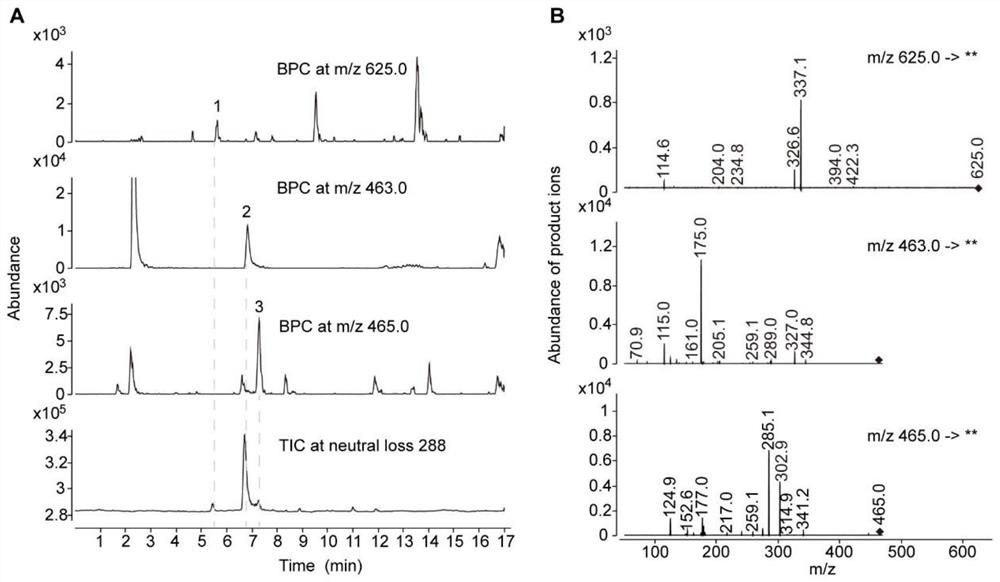
[0082] Example 2 Confirms the specific structure of the substance whose parent ion information is m / z 463
[0083] In order to accurately infer the structure of the m / z 463 substance under low-resolution mass spectrometry, in this example, UHPLC-QToF was used to perform high-resolution scanning at m / z 100-1700 of tds4-4 mutant and wild-type Arabidopsis samples (MS1 scan ).
[0084] The UHPLC-QToF detection instrument was an Agilent 1290Infinity II UPLC liquid chromatography coupled with an Agilent6546Q-ToF mass spectrometry system, equipped with an Agilent Zorbax RRHD SB (3×150mm, 1.7μm, C18) chromatographic column. The elution program of liquid chromatography is: phase A (ultrapure water containing 0.1% formic acid), phase B (acetonitrile containing 0.1% formic acid), flow rate 0.4 mL / min; program: 0-1 min, 5% B; 1- 2 min, 5-10% B; 2-17 min, 10-31% B; 17-18 min, 31-95%. The parameters of the mass spectrometer detector are: sheath gas flow rate 10L / min, temperature 400°C; dr...
Embodiment 3
[0094] Example 3 Standard synthesis of 4-(O-ascorbic acid)-(+)-catechin
[0095] According to the results of Example 2, this example adopts 3,4-trans leucocyanidin and L-ascorbic acid to prepare 4-(O-ascorbic acid)-(+)-catechin; the specific preparation process is as follows:
[0096] (1) After mixing 500 μM 3,4-trans leucocyanidin with an equimolar L-ascorbic acid aqueous solution, adjust the pH of the solution system to 7.4 with NaOH; 3,4-trans leucocyanidin It can be obtained by chemical synthesis, or the finished product can be purchased.
[0097] (2) The mixed solution system was placed in the dark at 30°C for 1 hour.
[0098] (3) using ethyl acetate to extract the solution system after the reaction in step (2), and retaining the water phase after extraction; wherein, the volume of ethyl acetate used for extraction is 3 times that of the solution system.
[0099] (4) HPLC separation and preparation; the Agilent 1200 HPLC system was equipped with an Agilent Zorbax SB (9....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com