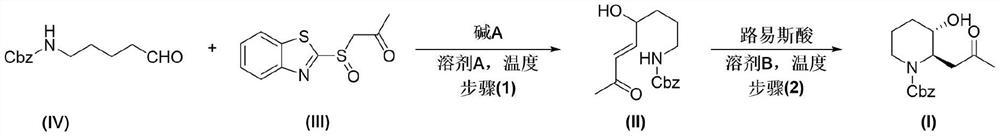
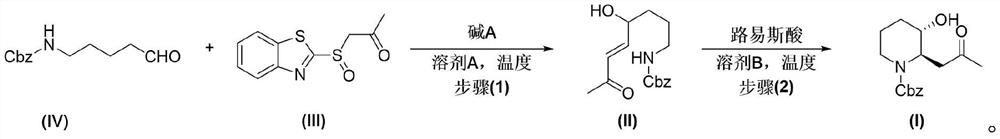
Preparation method of halofuginone intermediate trans-N-carbobenzoxy-(3-hydroxy-2-piperidyl)-2-acetone
A benzyloxycarbonyl and intermediate technology, which is applied in the field of pharmaceutical synthesis, can solve the problems of high cost, high price of Grubbs reagent, high price, etc., and achieves the effects of stable quality, reduced labor protection intensity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of compound IV:
[0072] Compound VIII:DIBAL-H:tetrahydrofuran=1:1.2:56.3.
[0073] (1) In a 20 mL three-necked flask, compound VIII (300 mg, 1.3 mmol) was dissolved in dry tetrahydrofuran (6 mL), the temperature was controlled to -78°C, and DIBAL-H (1 M, 1.54 mL, 1.54 mmol) was slowly added dropwise, and the temperature was kept warm. The reaction was carried out for 1 hour, and the reaction was monitored by TLC until the end of the reaction. Saturated ammonium chloride solution (10 g) was added and stirred to form a white solid layer. The white solid layer was diluted with ethyl acetate, filtered through a pad of celite, the filtrate was extracted with ethyl acetate, and the organic phase was dried over anhydrous sodium sulfate, spin-dried, and used directly for the next reaction.
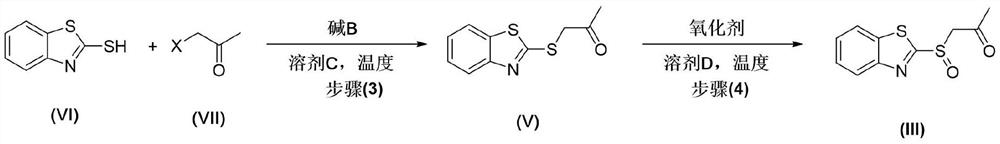
[0074] Preparation of Intermediate V:
[0075] Compound VI: bromoacetone: potassium carbonate: N,N-dimethylformamide=1:1.2:1.2:23.0.
[0076] (2) In a 2L three-necked flask...
Embodiment 2
[0095] Preparation of compound IV:
[0096] Compound VIII:DIBAL-H:tetrahydrofuran=1:1.2:56.3.
[0097] (1) In a 20 mL three-necked flask, compound VIII (310 mg, 1.3 mmol) was dissolved in dry tetrahydrofuran (6 mL), the temperature was controlled to -40°C, and DIBAL-H (1 M, 1.54 mL, 1.54 mmol) was slowly added dropwise, and the temperature was kept warm. The reaction was carried out for 1.5 hours, and the reaction was monitored by TLC until the end of the reaction. Saturated ammonium chloride solution (10 g) was added and stirred to form a white solid layer. The white solid layer was diluted with ethyl acetate, filtered through a pad of celite, the filtrate was extracted with ethyl acetate, and the organic phase was dried over anhydrous sodium sulfate, spin-dried, and used directly for the next reaction.
[0098] Preparation of Intermediate V:
[0099] Compound VI: bromoacetone: potassium carbonate: tetrahydrofuran=1:1.2:1.2:22.8.
[0100] (2) In a 2L three-necked flask, c...
Embodiment 3
[0111] Preparation of compound IV:
[0112] Compound VIII:DIBAL-H:tetrahydrofuran=1:1.2:56.3.
[0113] (1) In a 20mL three-necked flask, compound VIII (310mg, 1.3mmol) was dissolved in dry tetrahydrofuran (6mL), the temperature was controlled to -30°C, and DIBAL-H (1M, 1.54mL, 1.54mmol) was slowly added dropwise, and the temperature was kept warm. The reaction was carried out for 1.5 hours, and the reaction was monitored by TLC until the end of the reaction. Saturated ammonium chloride solution (10 g) was added and stirred to form a white solid layer. The white solid layer was diluted with ethyl acetate, filtered through a pad of celite, the filtrate was extracted with ethyl acetate, and the organic phase was dried over anhydrous sodium sulfate, spin-dried, and used directly for the next reaction.
[0114] Preparation of Intermediate V:
[0115] Compound VI: bromoacetone: potassium tert-butoxide: N,N-dimethylformamide=1:1.2:1.2:25.0.
[0116] (2) In a 2L three-necked flask...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com