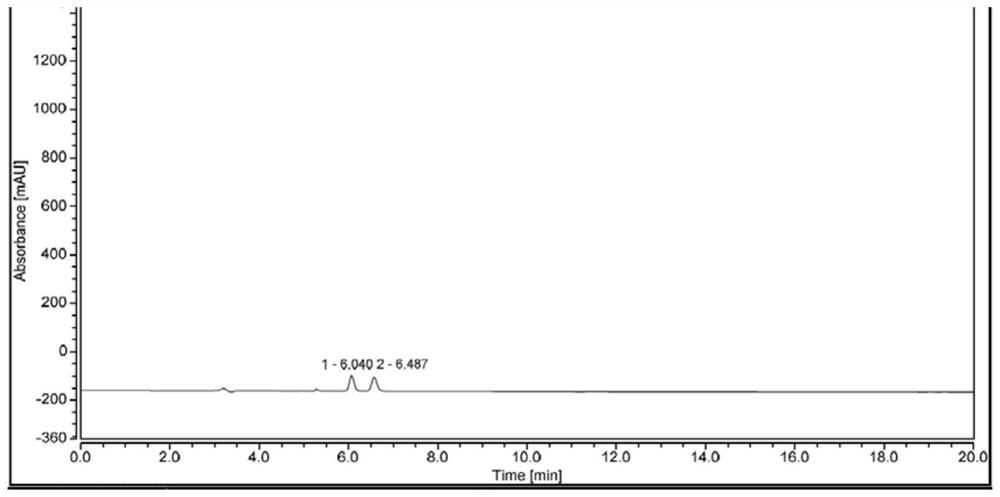
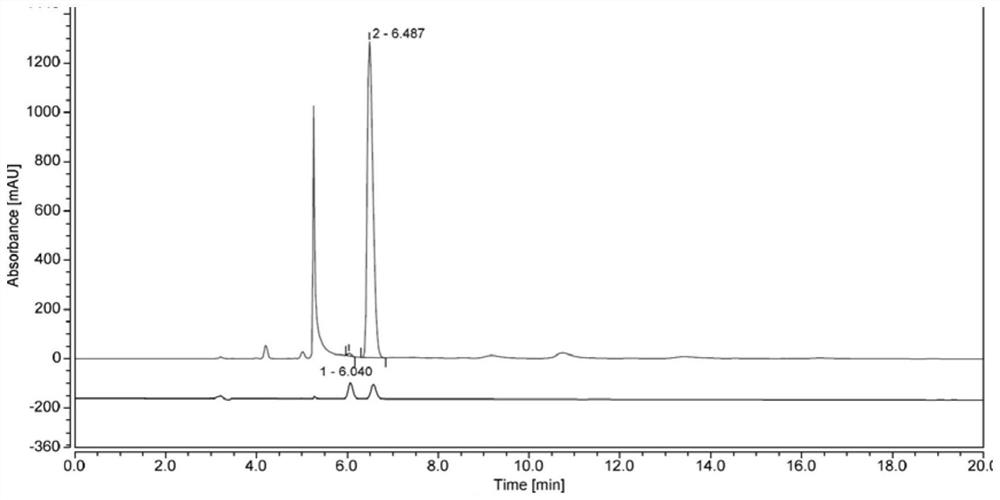
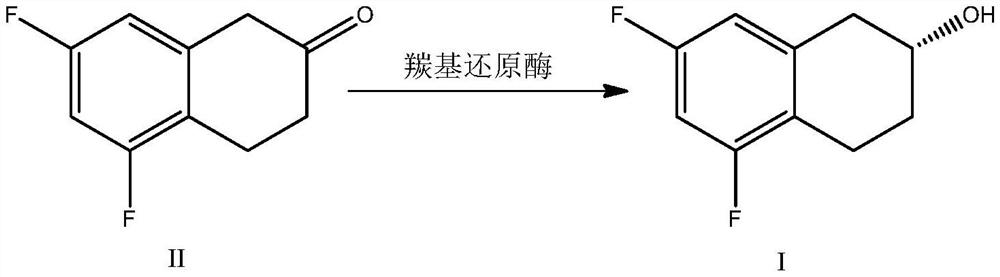
Biocatalysis preparation method of chiral tetrahydro-2-naphthol compound
A biocatalysis and tetrahydronaphthalene technology, applied in the field of medicine, can solve the problems of harsh reaction conditions, many reaction steps, low optical purity and the like, and achieve the effects of low cost, short reaction steps and high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Example 1: Preliminary Sieve of Carbonyl Rebutase
[0160] In this example, there is no preliminary screening of no less than 10 carbonyl reductase, wherein
[0161] ADH comes from Corynebacterium Sp.st-10, and its genbank serial number is AB190261;
[0162] LSADH comes from Leifsonia Sp.strain S749, its GenBank serial number is AB213459;
[0163] ARQR comes from Agrobacterium Radiobacter ECU 2556, its genbank serial number is WP_015918093.1;
[0164] Fusc2 comes from Fusidioides Strain ATCC 14700, and its genbank serial number is QBB00668.1;
[0165] LK comes from Lactobacillus Kefir, its genbank serial number is WP_054768785.1;
[0166] BD originated from Brevundimonas Diminuta 470-4, and its GenBank serial number is eky29933.1;
[0167] CK20 is derived from Chryseobac-Terium Sp.ca49, and its GenBank serial number is AHC30858.1;
[0168] Fusc1 comes from Fusidioides Strain ATCC 14700, and its genbank serial number is QBB00667.1;
[0169] DHCR comes from Debaryomyces Hanse...
Embodiment 2
[0177] Example 2: Construction of Carbonyl Reprotor Enzyme Engineering
[0178] The carbonyl reductase (LSADH) gene from Leifsonia Sp.strain S749 was brought to a PET28A (+) vector, transferred to E. coli DH5α sensitic cells, kanamycin resistant plate culture. After challenging the positive transformant single colonies and extracts the plasmid sequence, the recombinant plasmid is extracted, and then the host E. coli BL21 (DE3), plate culture, sequential colonies, lb culture, and obtain a recombinant gene engineering of carbonyl reductase (LSADH). bacteria.
Embodiment 3
[0179] Example 3: Construction and screening of carbonyl reduced enzyme mutant libraries
[0180] With wild-type carbonyl reductase LSADH as a template, using a random dot mutant kit ( Iisite-Directed Mutagenesiis Kit is erroneous PCR, or mutated wild-type carbonyl reductase gene LASDH by oriented evolution to obtain a plasmid library comprising an evolutionary carbonyl reductase gene. The constructed plasmid library was transferred to E. coli BL21 (DE3) (item number: Kang as century CW0809S), in an oven at 37 ° C, cultured overnight in LB solid medium containing 50 μg / ml kanamycin. Single colonies were selected to a 96-well plate containing 400 μl of LB liquid medium (containing 50 μg / ml kanamycin), 37 ° C, 200 rpm overnight culture, and obtained seed fluid. 10 μl of seed fluid was then transferred to a 96 deep hole plate containing 400 μl of fermentation medium (containing 50 μg / ml kanamycin) to the OD600 value> 0.8 at 37 ° C. Then, an ultra-concentration of 1 mm isopropy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com