Preparation method of cyclic carbonate and preparation method of sulfonamide bifunctional quaternary ammonium salt catalyst
A cyclic carbonate and catalyst technology, applied in the field of preparation of cyclic carbonate, can solve the problems of poor selectivity, oxidation, and easy hydrolysis, etc., and achieve the effects of improving reaction efficiency, avoiding toxicity, and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
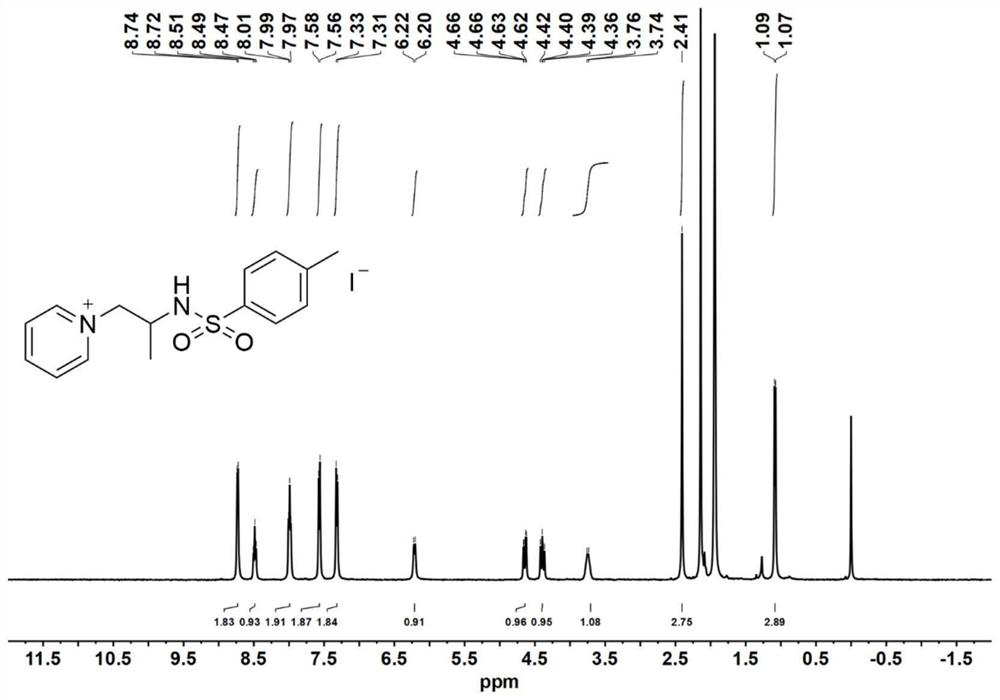
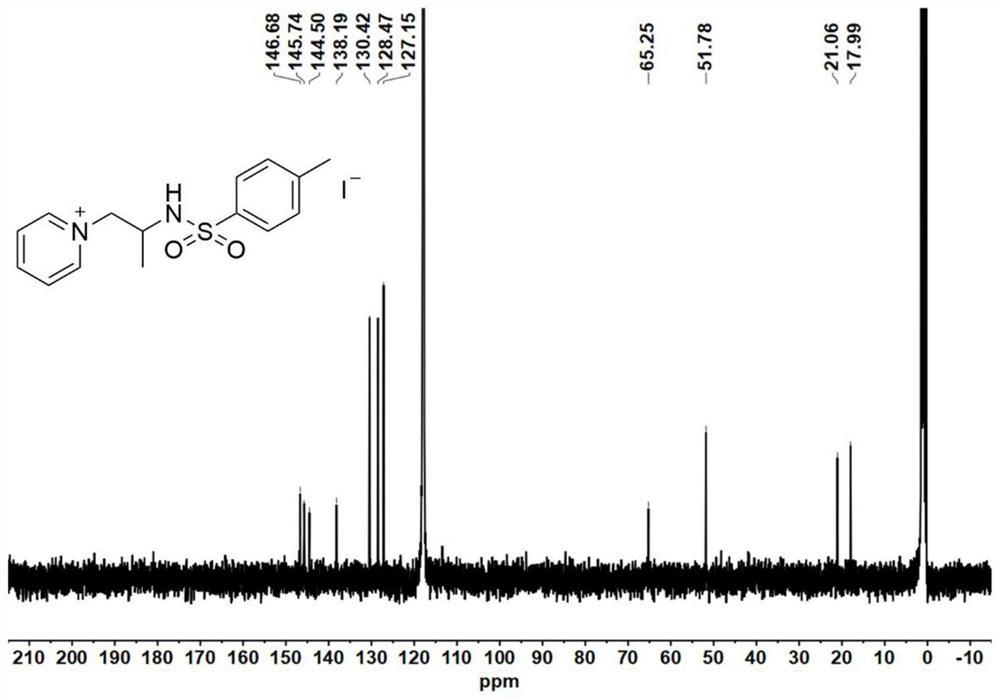
[0087] Catalyst precursor - synthesis of aziridine:
[0088] Add 4-dimethylaminopyridine (DMAP, 0.1 equiv) to a solution of glycinol (20 mmol) and triethylamine (TEA, 3.5 equiv.) in anhydrous acetonitrile (120 mL) at 0°C; then slowly add p-toluenesulfonyl chloride (2.0equiv.), keep the reaction liquid milky white during the process. After the p-toluenesulfonyl chloride was added dropwise, the reaction mixture was stirred for 30 minutes to warm up to room temperature, and the reaction was continued for 5-7 hours. After the reaction was completed, the reaction solution was spun out of the solvent to obtain a light yellow emulsion, which was then dissolved with 200 mL of ethyl acetate. Wash the organic phase with saturated sodium chloride solution (80 mL) 3 times, and dry the organic phase with anhydrous magnesium sulfate overnight, remove the anhydrous magnesium sulfate by filtration, and concentrate the filtrate by rotary evaporation. The obtained crude product was purified b...
Embodiment 2
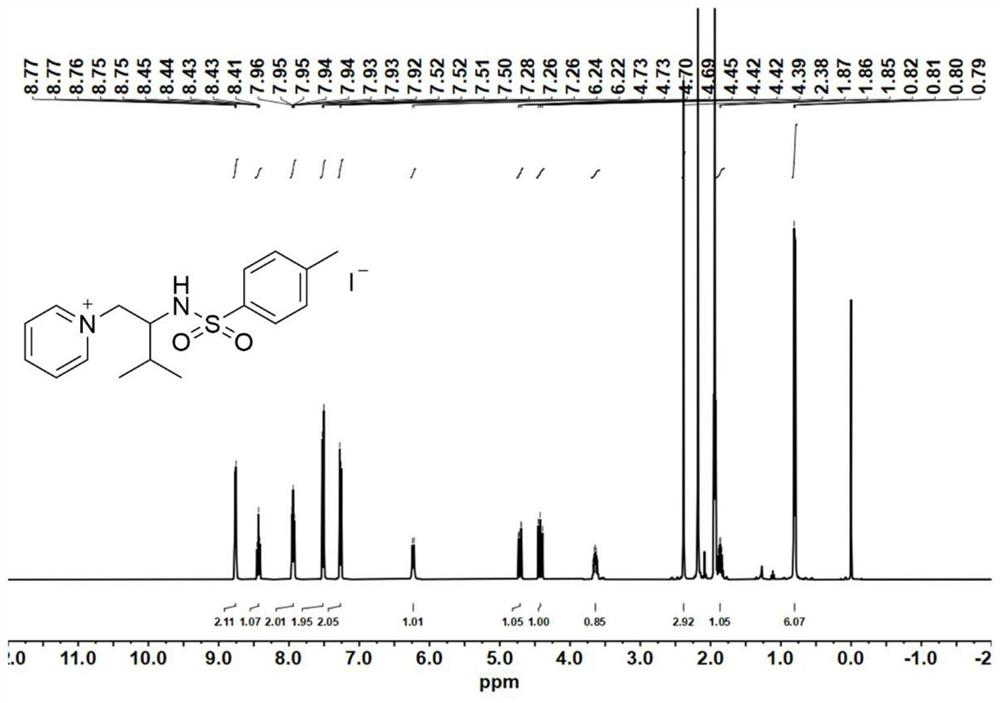
[0094] Catalyst precursor - synthesis of aziridine:
[0095] At 0°C, add 4-dimethylaminopyridine (DMAP, 0.1 equiv) to a solution of valinol (20 mmol) and triethylamine (TEA, 3.5 equiv.) in anhydrous acetonitrile (120 mL); then slowly drop p-toluenesulfonyl chloride (2.0equiv.), keep the reaction liquid milky white during the process. After the p-toluenesulfonyl chloride was added dropwise, the reaction mixture was stirred for 30 minutes to warm up to room temperature, and the reaction was continued for 5-7 hours. After the reaction was completed, the reaction solution was spun out of the solvent to obtain a light yellow emulsion, which was then dissolved with 200 mL of ethyl acetate. Wash the organic phase with saturated sodium chloride solution (80 mL) 3 times, and dry the organic phase with anhydrous magnesium sulfate overnight, remove the anhydrous magnesium sulfate by filtration, and concentrate the filtrate by rotary evaporation. The obtained crude product was purified ...
Embodiment 3
[0101] Catalyst precursor - synthesis of aziridine:
[0102] At 0°C, to phenylglycinol (20mmol) and K 2 CO 3 (4.0 equiv.) Add anhydrous acetonitrile (120mL) to dissolve; then slowly add p-toluenesulfonyl chloride (dissolve in anhydrous acetonitrile) (2.0equiv.), keep the reaction liquid milky white during the process. After the p-toluenesulfonyl chloride was added dropwise, the reaction mixture was stirred for 30 minutes to warm up to room temperature, and the reaction was continued for 5-7 hours. After the reaction was completed, the reaction solution was spun out of the solvent to obtain a white emulsion, and then toluene was added dropwise (stopped when the organic layer and the solid layer were separated). The obtained crude product was purified by silica gel column chromatography (petroleum ether / ethyl acetate=10 / 1) to finally obtain aziridine 33 as a white powder.
[0103] Catalyst Synthesis:
[0104] Dry the flask and add the rotor, then add aziridine 33 (3mmol), Yb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com