Exosome-loaded GelMA hydrogel microneedle as well as preparation method and application thereof
An exosome and hydrogel technology, applied in the fields of biomedicine and molecular biology, can solve the problems of poor treatment effect, low efficiency of exosome administration, and inability to achieve long-term stable release, so as to reduce the local microenvironment. , the effect of alleviating SCI complications and improving prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
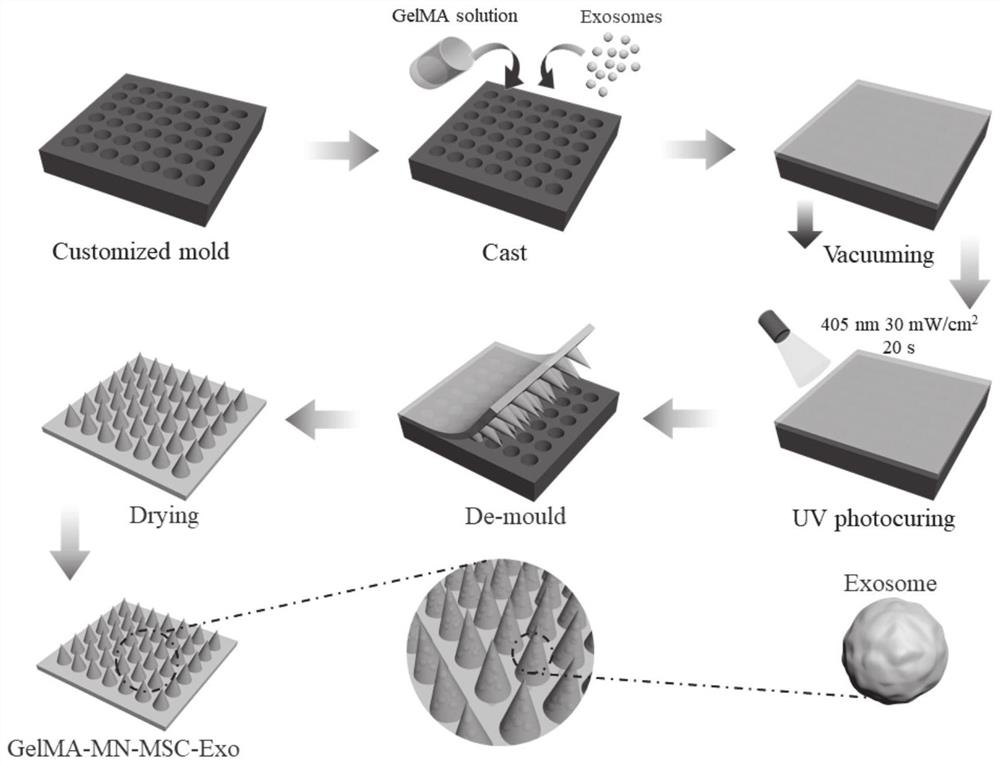
[0072] In yet another specific embodiment of the present invention, a method for preparing the above-mentioned microneedles is provided, the preparation method comprising:
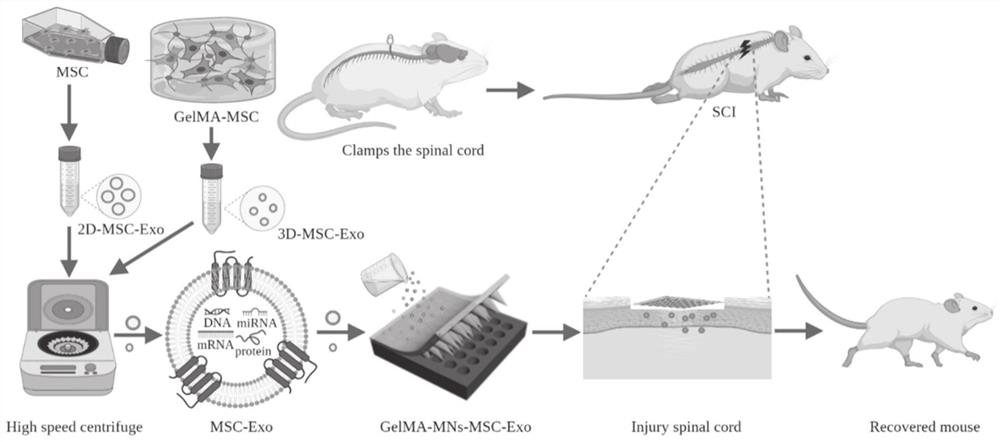
[0073] S1. Culture and preparation of exosomes;
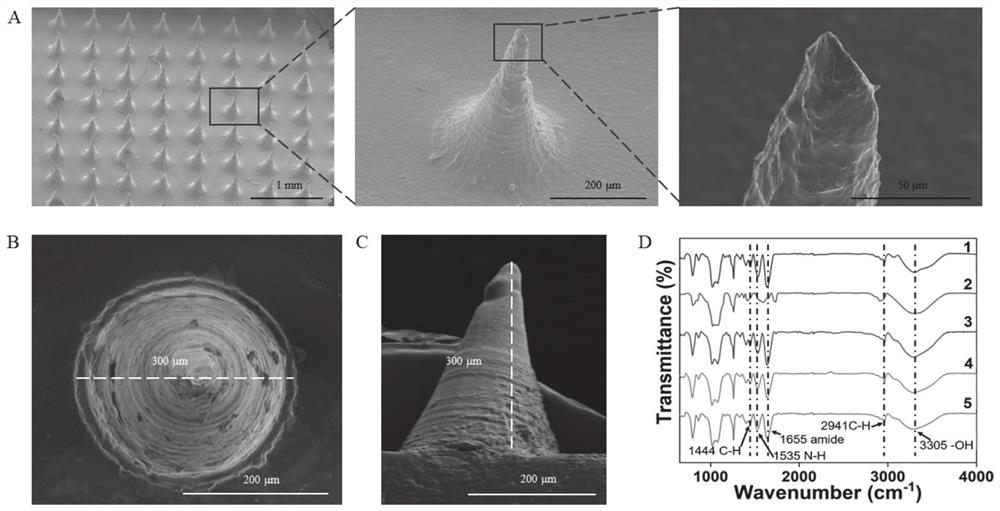
[0074] S2. Add the exosomes prepared in step S1 into the GelMA hydrogel precursor solution, and obtain it after photocuring.
[0075] Wherein, in the step S1, the specific method for culturing and preparing exosomes includes:
[0076] The mesenchymal stem cells were cultured in 2D or 3D, and the exosomes secreted by the mesenchymal stem cells were collected by centrifugation.
[0077] In yet another specific embodiment of the present invention, the 3D culture specifically includes: adding mesenchymal stem cells into the GelMA precursor solution, adding the cell solution into the curing ring, adding complete medium after photocuring, and short-term (such as 1 -10min, preferably 5min) after culturing, remove the culture medium, then add a new culture medium...
Embodiment
[0092] 1. Experimental animals: Adult male SPF grade SD rats weighing 280-320 g were selected for this study, and the experimental animals were purchased from Jinan Pengyue Experimental Animal Breeding Co., Ltd. They were kept in the SPF-grade animal breeding room of the Animal Center of the First Affiliated Hospital of Shandong First Medical University, with a temperature of 20±2°C and a natural light / dark (≈12h-12h) cycle. Animal experiment protocols were approved by the Animal Care and Use Committee of Shandong First Medical University according to the principles outlined in the Animal Care and Use Guide. Personnel working with animal models received systematic training in accordance with Institutional Animal Care and Use Committee guidelines (IACUC).
[0093]2. Rat SCI model establishment: The spinal cord injury model was established by the clamp method. The specific method was: all rats were fasted for 6 hours before operation, anesthetized with isoflurane, and the dose o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com