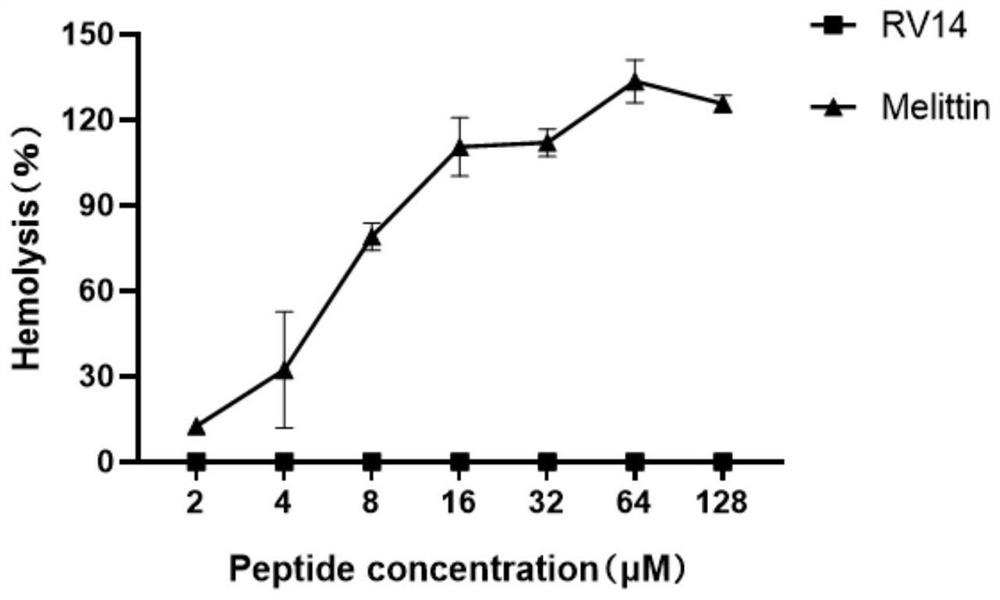
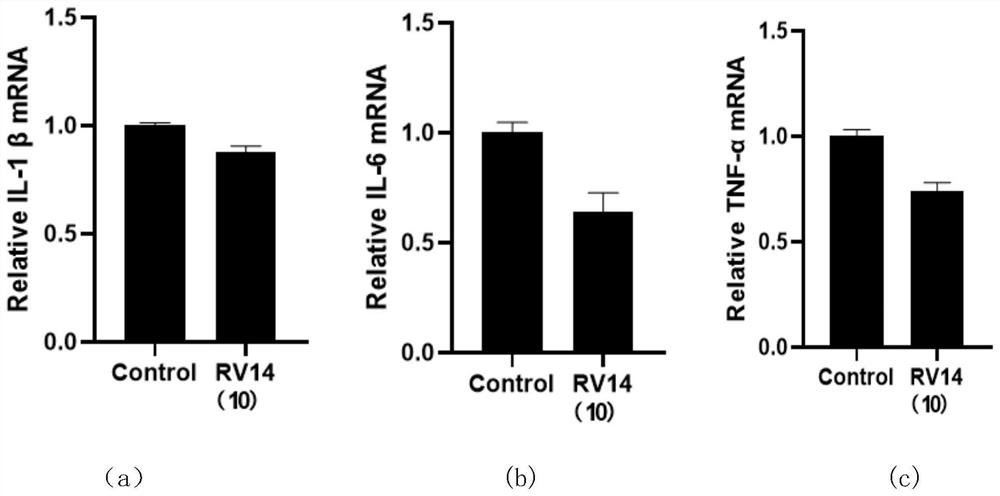
A defensin-like immunomodulatory tetradeceptide rv14 and its preparation method and application
An immune regulation, RV14 technology, applied in the biological field, can solve the problem of no antibacterial activity, achieve good antibacterial activity, do not cause hemolysis, and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1 Design and synthesis of antimicrobial peptides
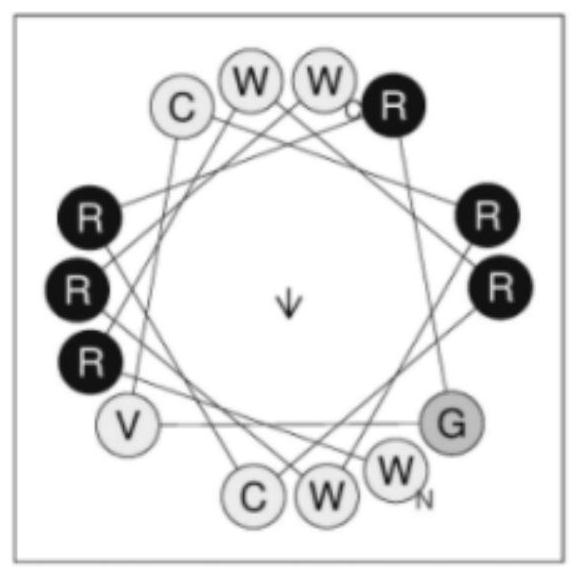
[0014] Step 1: Through the alignment of defensin sequences, a central skeleton CxxGyC with defensin characteristics is obtained, where x is a cationic amino acid, y is a hydrophobic amino acid, and the immunomodulatory characteristics of beta defensin are retained as much as possible, and the skeleton sequence is short. , which is more conducive to practical synthesis and application. Through the screening of therapeutic index, RV14 with the highest therapeutic index was selected from the 14 peptides as the target peptide. Its skeleton is CRRGVC.
[0015] Step 2: Add WRWR and RWRW amino acid sequences to the N- and C-termini of the backbone. This fragment has good antibacterial activity, which makes it have good antibacterial activity, and at the same time, it is amidated at the C-terminus to increase its cationicity and stability. The final sequence is WRWRCRRGVCRWRW-NH 2 .
[0016] Step 3: Antibacterial p...
Embodiment 2
[0020] Example 2 Secondary structure analysis of antimicrobial peptides
[0021] The secondary structures of antimicrobial peptides were analyzed by CD spectroscopy. The results showed that in the water environment, the peptide RV14 assumed a β-sheet structure and still retained the β-defensin fold structure. It is proved that the designed peptide has a strong structure, which is different from other peptides showing random coil structure in water, RV14 can show structure even in water. Means it can also function in water. In contrast, in the hydrophobic environment (50% TFE) mimicking microbial membranes and in the negatively charged prokaryotic membrane environment (30 mM SDS), the β-sheet structure of the peptides was weaker. Most antimicrobial peptides exhibit random coils in the aqueous environment, but exhibit structures in the cell membrane environment.
Embodiment 3
[0022] Example 3 Salt ion stability of antimicrobial peptides
[0023] Salt ions are added to the BSA solution to configure BSA solutions with different salt ions. And the antibacterial activity was measured again according to the method of antibacterial activity. The final concentrations of salt ions measured were: 150 mM NaCl, 4.5 mM KCl, 6 mM NH 4 Cl, 8mM ZnCl 2 , 1mM MgCl 2 , 2.5mM CaCl 2 and 4mM FeCl 3 . The results are shown in Table 2, the antimicrobial peptides have good stability in the seven salt ions. Except in the sodium ion environment, the antibacterial effect was slightly weakened, and the antibacterial activity remained unchanged in the other six salt ion environments.
[0024] Table 2 Antibacterial activity of RV14 against bacteria in salt ion environment
[0025]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com