Betulinic acid derivative as well as preparation method, pharmaceutical composition and application thereof
A technology of betulinic acid and its derivatives, applied in the field of medicine, can solve the problems of low bioavailability, in vivo transmission, metabolism, and poor solubility, and achieve excellent anti-tumor activity, high yield, and good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: the preparation of compound
[0086] Preparation of compound ZX-6 adopts the following method:
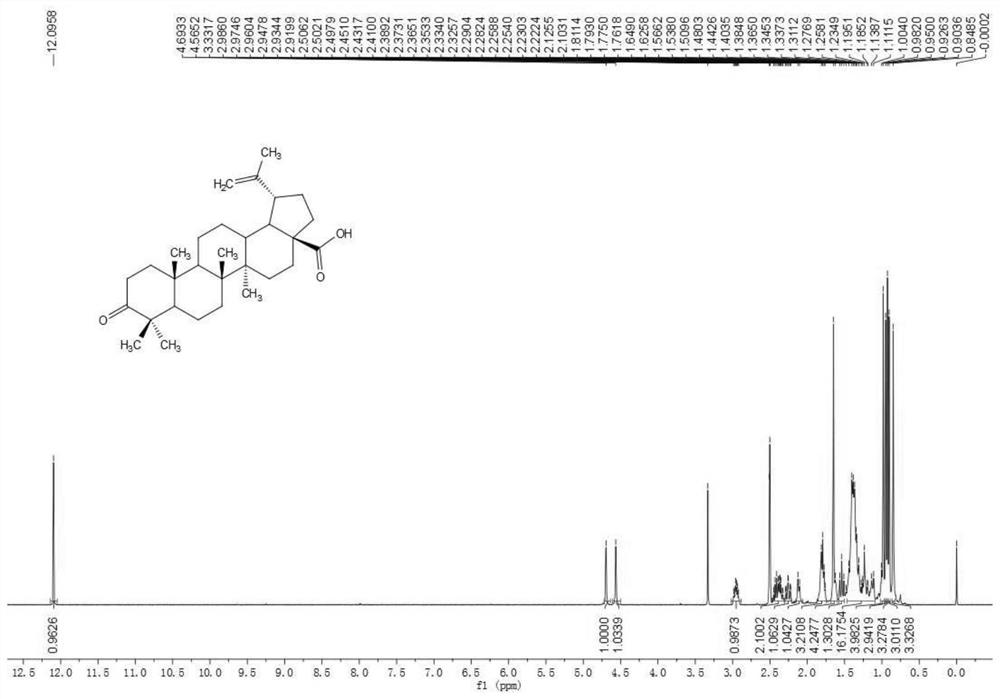
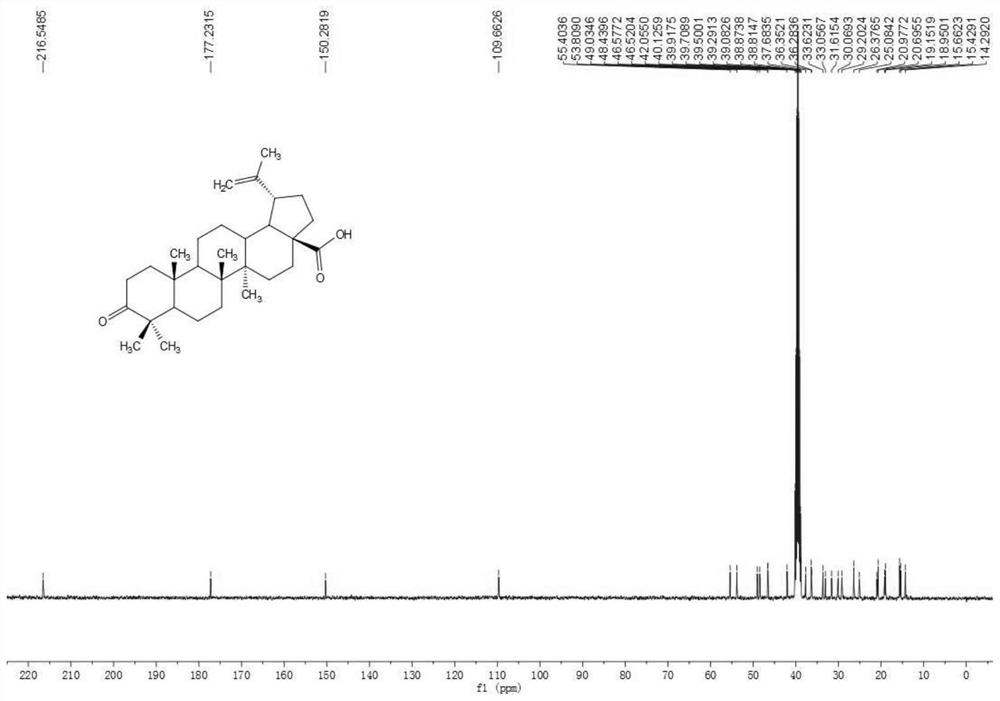
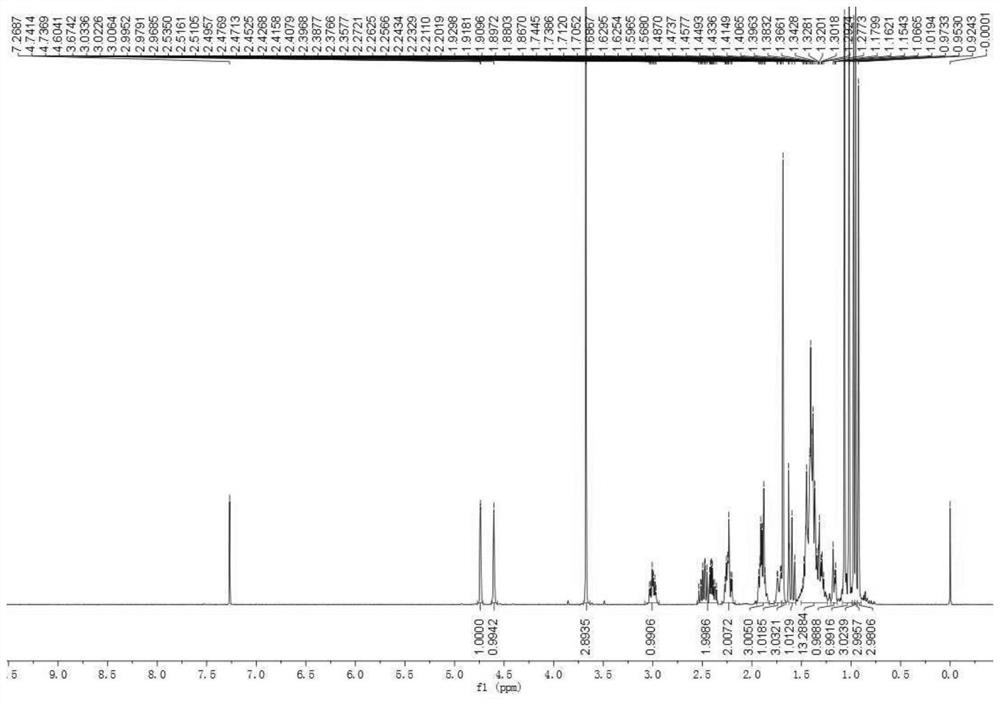
[0087] a. First use Jones reagent to oxidize betulinic acid to betulinic acid compound 2.
[0088] b. Using oxalyl chloride and methanol to esterify the betulinic acid compound 2 prepared in step a) into betulinic acid methyl ester compound 3.
[0089] c. using propyne bromide to react with betulinate methyl ketoate compound 3 prepared in step b) to obtain compound 4.
[0090] d. Using sodium borohydride to reduce the carbonyl group in compound 4 prepared in step c) to hydroxyl to prepare compound 5.
[0091] e. using lithium iodide to demethylate the compound 5 prepared in step d) into a carboxylic acid, and prepare compound 6.
[0092] f. Adding 4'-azacytidine to the betulinic acid compound 6 prepared in step e) through a click reaction to synthesize the betulinic acid derivative ZX-6.
[0093]
[0094] Concrete synthetic route is as follows:
[0095]...
Embodiment 2
[0105] Embodiment 2: biological activity test
[0106] 2.1 The inhibitory activity test of the compound of the present invention to Hela, C33a, Siha cells
[0107] In this experiment, different concentrations of test compounds were used to act on human cervical cancer cell lines Hela, C33a, and Siha cells for 48 hours, and the inhibition rate of cell growth was detected by MTT method to detect the inhibition degree of compounds on different tumor cells.
[0108] experimental method:
[0109] Dissolve the subject in a certain volume of DMSO, make a mother solution with a concentration of 10mmol / L and store it at 4°C. When used, take the mother solution and dilute it to a certain multiple solution.
[0110] The specific operation steps are as follows:
[0111] (1) Take Hela, C33a, and Siha cells in the logarithmic growth phase, centrifuge, resuspend, and adjust the cell suspension density to 8×10 4 cell / mL, inoculate in 96-well culture plate, add 100 μL of cell suspension to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com