1,4,6-trihydroxy-8-branched-chain-9,10-anthraquinone compound and application thereof in preparation of bacteriostatic agent
A compound, anthraquinone technology, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
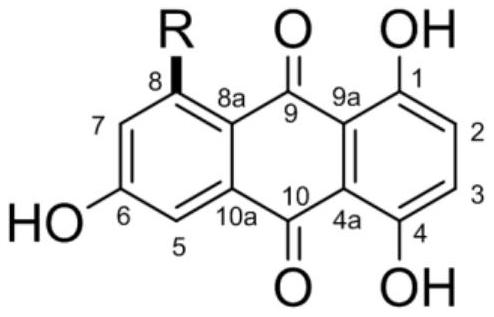
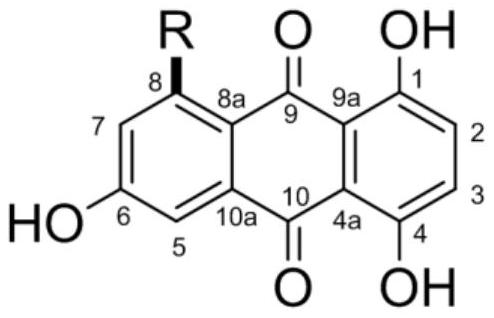
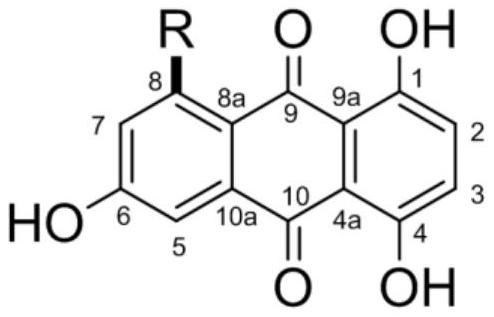
[0023] 1,4,6-trihydroxy-8-branched-9,10-anthraquinone compound, the structural formula of the compound is as follows:
[0024]
[0025] Among them, R is: propyl, butyl, isopentyl, pentyl, pentylcarboxy, 2'-carbonylpentyl, 2'-carbonylhexyl, hexyl, 2'-hydroxypropyl, 4'-hydroxyisopentyl Or 2'-hydroxypentyl.
[0026] When the above-mentioned R is a different group, they are named as compound 1-11 in sequence. Specifically, the structural formula of compound 1-11 is as follows:
[0027]
[0028] Among them, the applicant of compound 1-8 has reported that the UV, HR-ESI-MS and NMR spectral data of the structural identification of compound 9-11 are as follows:
[0029] Compounds 9-11 are all red powders, and their common UV absorption spectrum (466, 277, 225 nm) indicates that these compounds have a common 1,4,6,8-substituted anthraquinone skeleton.
[0030] HR-ESI-MS data: compound 9 (m / z337.0688, [M+Na] + ) deduces that its molecular formula is C 17 h 14 o 6 . Compound...
Embodiment 2
[0037] Compound 1-11 antibacterial activity test:
[0038] Select Staphylococcus aureus, Erysipelothrix rhusiopathiae, Streptococcus suis, Escherichia coli and Pseudomonas aeruginosa for subculture on nutrient agar slant medium Once, inoculate it into the nutrient broth medium and culture it at 37°C for 6-12 hours, and place it in the refrigerator for later use. Take the sample to be tested and the positive control (streptomycin and penicillin), and dilute to 100 μg / mL, 50 μg / mL, 25 μg / mL, 12.5 μg / mL, 6.25 μg / mL, 3.125 μg / mL, 1.56 μg / mL and 0.78μg / mL, shake and mix, transfer 1mL to a 96-well plate, use the culture medium as the blank control, select 3 replicates for the blank and each concentration, repeat the experiment 3 times, and incubate at 37°C for 12-18h. The absorbance was measured with a microplate reader at 630 nm to determine the MIC value.
[0039] Results: Compounds 1, 2, 4, 5, 8 and 10 had significant antibacterial activity against Staphylococcus aureus (MIC<0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com