A liquid-phase method for determining the content of cellulose in excipients in medicine
A technology of cellulose content and liquid phase method, which is applied in the directions of measuring devices, material separation, and material analysis, etc., which can solve the problems of unable to meet the monitoring requirements of cellulose content limit, low detection accuracy, cumbersome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The method for determining the cellulose content of the auxiliary material in the liquid phase method comprises the steps:
[0033] 1. Liquid chromatography conditions: Chromatographic column: TSKgtl G2000SW column, 60cm*7.5mm, 10μm; detector: charged aerosol detector; mobile phase: 10mmol / L ammonium acetate solution (pH 4.5): acetonitrile=95:5; Atomization temperature: 50°C; flow rate: 1.0ml / min; injection volume: 10μl.
[0034] 2. Reference substance solution: Accurately weigh 25.79 mg of hydroxyethyl cellulose reference substance, accurately weigh it, put it in a 50ml measuring bottle, add warm water (60°C) to dissolve it, add water to dilute to the mark, and shake well to obtain hydroxyethyl cellulose Ethyl cellulose reference substance stock solution. Then precisely pipette 5ml of the hydroxyethyl cellulose reference substance stock solution into a 10ml volumetric flask, dilute with water to the mark, and shake well to obtain the reference substance solution.
[...
Embodiment 2
[0039] The method for the determination of the cellulose content of excipients in drugs by liquid phase method was validated, and the validation results are as follows:
[0040] 1. Specificity: Get solvent water, reference substance solution, negative blank solution, need testing solution and standard-added need testing solution respectively and detect by the chromatographic conditions of embodiment 1, record chromatogram, investigate method specificity.
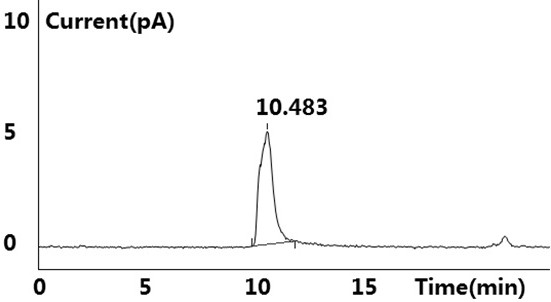
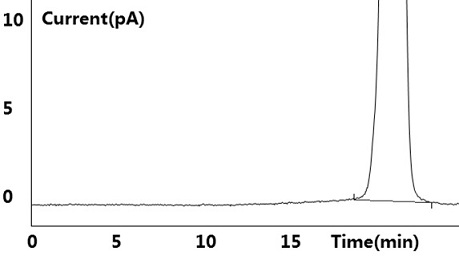
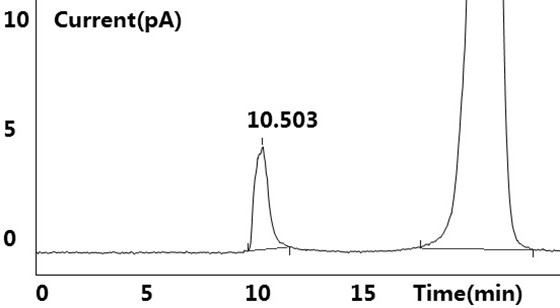
[0041] see results Figure 1~2 Shown, blank solution and negative blank solution ( figure 2 ) did not detect the target peak, no interference to the detection; the reference substance solution showed the target peak, and no other impurity peaks were detected ( figure 1 ); the test solution ( image 3 ) and the retention time of the target peak in the chromatogram of the standard-added test solution is consistent with the retention time of the main peak in the reference solution (retention time is between 10.483min~10.515m...
Embodiment 3
[0058] Detect reference substance solution and need testing solution respectively with reference to the method condition of embodiment, difference only is to adjust the pH of ammonium acetate solution to be 4.3 and 4.7, record chromatogram, calculate the hydroxyethyl cellulose concentration in reference substance solution and need testing solution content. The results showed that the pH value of the mobile phase fluctuated in the range of 4.3-4.7, the RSD of the peak area of hydroxyethyl cellulose in the reference solution for 6 consecutive times was between 0.4% and 2.1%, and the RSD of the retention time was between 0.2 and 0.4%. The RSD of the hydroxyethyl cellulose content measured in the test solution was 2.7%, and the durability result met the requirements.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com