Method for improving acid stability of glucose oxidase, mutant Q241E/R499E, gene and application
A technology of glucose oxidase and mutants, applied in the direction of oxidoreductase, microbial-based methods, biochemical equipment and methods, etc., can solve the problem of low enzyme activity, achieve improved acid stability, enhanced acid stability, improved The effect of acid stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1, recombinant strain GS115 (pPIC9 -godm10 ) preparation
[0053] (1) Amplify the nucleic acid sequence of the maternal high thermostable glucose oxidase mutant GODM10 godm10
[0054] Amplified by PCR godm10 Gene fragments, the vector pPIC9 nucleic acid fragments were obtained by double enzyme digestion, and the two were connected by a recombination kit to obtain the recombinant plasmid pPIC9- godm10 , and transform Pichia pastoris GS115 to obtain recombinant Pichia pastoris strain GS115 (pPIC9 -godm10 ). The primers used in PCR are as follows:
[0055] godm10-pPIC9-F (GOD ID No: 9, 40bp): GGTATTGAGGCTTCCTTGTTGACTGACCCAAAGGAGGTCG
[0056] godm10-pPIC9-R (GOD ID No: 10, 40bp): TTGCATGGAGGCGTAGTCAGCCAAAACAGCGTCTGCGATC
[0057] Among them, godm10-pPIC9-F and godm10-pPIC9-R are used to amplify the gene coding sequence of glucose oxidase M10; the vector pPIC9 is obtained by extracting the preserved strains after bottle culture. After the amplificati...
Embodiment 2
[0060] Embodiment 2, recombinant bacterial strain GS115 (pPIC9 -godm10-Q241E , pPIC9 -godm10-R499E , pPIC9 - godm10-Q241E / R499E ) preparation
[0061] (1) Recombinant plasmid pPIC9- godm10-Q241E , pPIC9- godm10-R499E , pPIC9- godm10-Q
[0062] 241E / R499E build
[0063] After optimization, the design of the mutation site is to mutate glutamine and arginine at positions 241 and 499 to glutamic acid respectively, introduce the mutation site through the method of point mutation kit, and perform sequencing verification on it to obtain glucose Oxidase mutant plasmid pPIC9- godm10-Q241E , pPIC9- godm10-R499E , pPIC9- godm10-Q241E / R499E . The primers used are as follows:
[0064] Q241E-F (God ID No: 10) AGACCTAACTTGGAGGTTTTGACCGGTCAATACGT
[0065] Q241E-R (God ID No: 11) ACCGGTCAAAACCTCCAAGTTAGGTCTTTGGTAGT
[0066] R499E-F (God ID No: 12) GATGCTGACTTGGAGGCTTGGGTTGAATACATTCC
[0067] R499E-R (God ID No: 13) TTCAACCCAAGCCTCCAAGTCAGCATCGTAGGCCA
[0068] Among t...
Embodiment 3
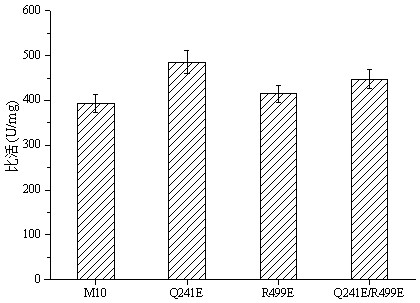
[0071] Example 3, the acquisition of high thermostable glucose oxidase M10 and Q241E, R499E, Q241E / R499E
[0072] 1. Induced expression of GODM10 and Q241E, R499E, Q241E / R499E
[0073] The obtained recombinant expression strain GS115 ( pPIC9-godm10 ) and GS115 (pPIC9- godm10-Q241E , pPIC9- godm10-R499E , pPIC9-godm10-Q241E / R499E ) were inoculated into YPD medium for seed culture, 200 rpm, 30°C for 48 h, then transferred to BMGY medium with 1% inoculum, 200 rpm, 30°C for 48 h, and collected after enriching enough bacteria Bacteria were added to BMMY medium containing 1% methanol to induce expression.
[0074] 2. Purification of GODM10 and Q241E, R499E, Q241E / R499E
[0075] Centrifuge the induced bacterial solution at 12000 rpm for 10 min, collect the supernatant and concentrate it, then dialyze it with 10 mM disodium hydrogen phosphate solution (adjust the pH to 6.5 with citric acid), and then carry out the ion exchange layer for the dialyzed enzyme For analysis, solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com