Reagent and kit for detecting Kawasaki disease and evaluating curative effect and application
A technology for detection kits and efficacy evaluation, applied in biological testing, disease diagnosis, measurement devices, etc., can solve problems such as lag and complex Kawasaki disease diagnosis, and achieve the effect of increasing sensitivity, improving medical capacity, and improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] CXCL10 and CCL23 ELISA plate preparation
[0054] In this embodiment, the leading reaction solution required for the ELISA reaction was prepared according to the specific components and conditions listed in Table 1 to Table 3 below. The CXCL10 coating solution and the CCL23 coating solution were added to different 96-well plates respectively; the coating solution was discarded, and the sealing solution was added to each well to seal; the sealing time was 2 hours at room temperature, and after 5 hours of air drying, together with The desiccant is stored in an aluminum foil bag and refrigerated.
[0055] Table 1 Preparation of CXCL10 coating solution components
[0056] components final concentration per reaction PBS buffer solution (pH=7.4) 1× CXCL10 monoclonal antibody 1μg / mL
[0057] Note: The commercial information of the CXCL10 monoclonal antibody is: manufacturer: invitrogen, product name: CXCL10 Polyclonal Antibody, product number: PA...
Embodiment 2
[0064] ELISA operation and result analysis
[0065] Then, perform the ELISA operation according to the following steps.
[0066] 1. Reagent preparation: Take out the components of the kit (including the microplate prepared in Example 1), and equilibrate for about 30 minutes at room temperature (18-25°C). After the microplate is opened, take the required amount, and the rest should be timely Seal up and store at 2-8°C.
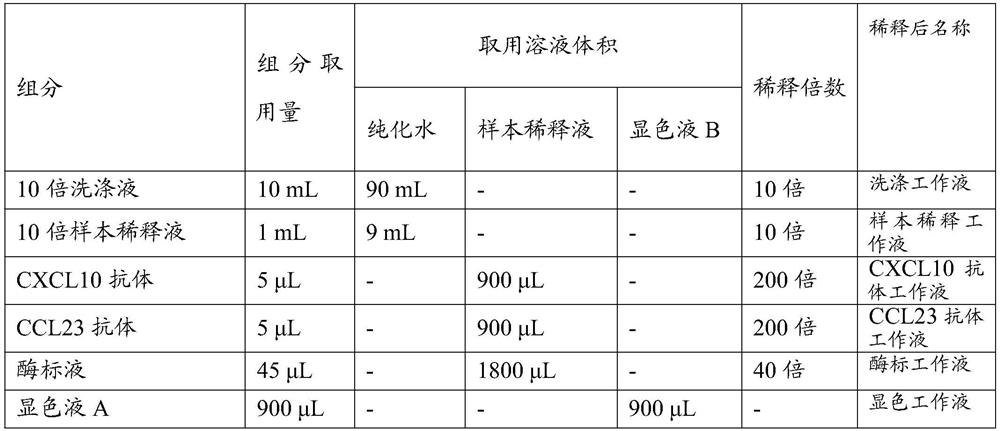
[0067] In this embodiment, the total number of test samples is 8 cases, and the samples are prepared according to the reagent composition list listed in Table 4. If the number of detected samples is 16 cases, then the components listed in Table 4 will be increased twice. and so on.
[0068] Table 4 Reagent Component Preparation Table
[0069]
[0070] Among them, the 10-fold sample dilution solution contains PBS buffer solution and BSA; the enzyme labeling solution is horseradish peroxidase; the 10-fold washing solution contains PBS buffer solution and T...
Embodiment 3
[0099] Reagent Performance Verification
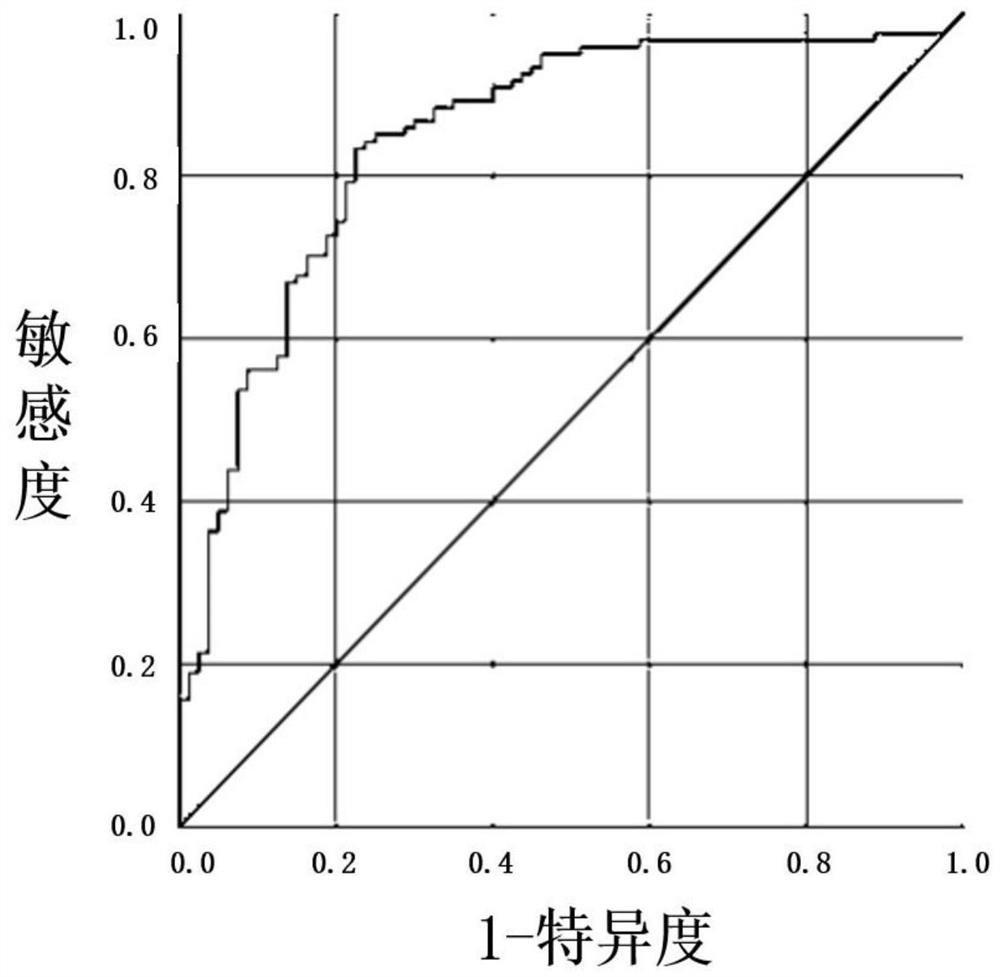
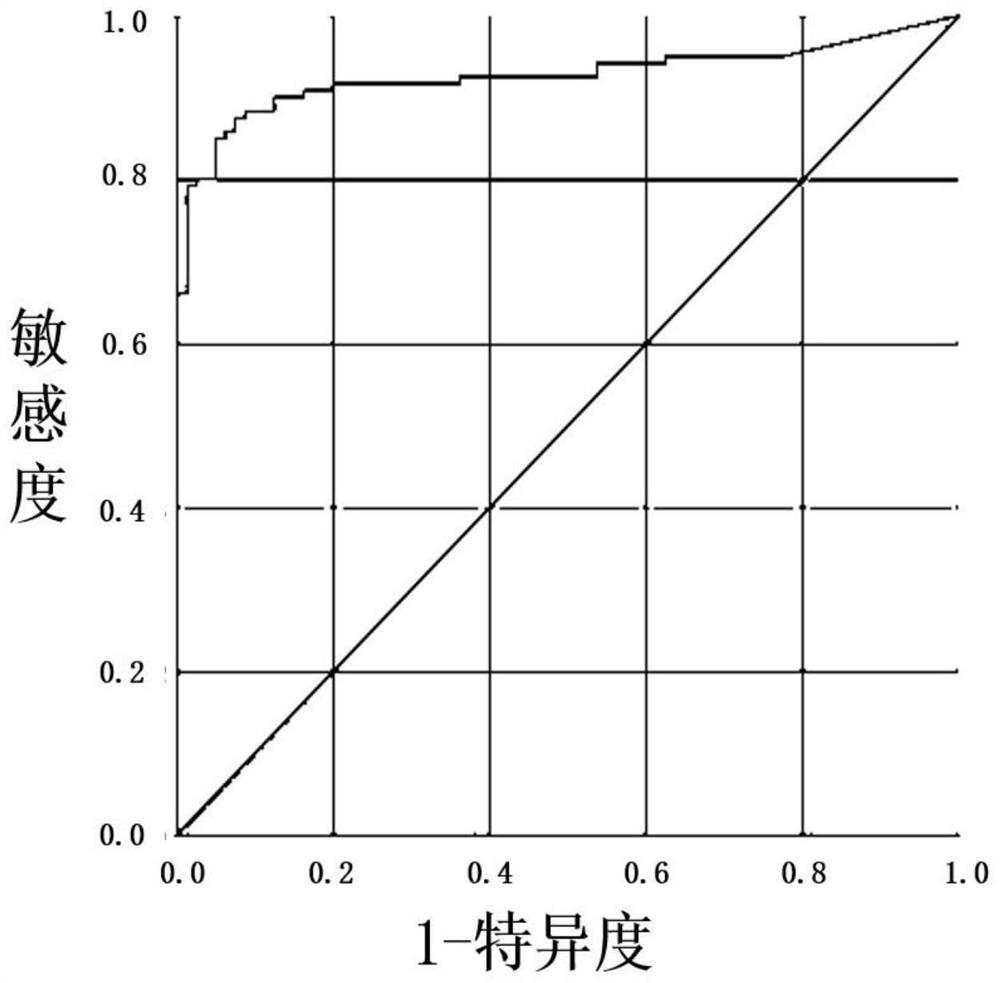
[0100] In this embodiment, 10 existing positive reference products and 4 negative reference products were obtained, and double verification was carried out on the detection reagent of the present invention and the aforementioned judgment methods. The results show that the detection reagent of the present invention and its judgment criteria all meet the positive and negative requirements of the reference product (Table 7 and Table 8).
[0101] Table 7-1 Concentration and Judgment Results of Positive Reference Substances CXCL10 and CCL23
[0102]
[0103] Table 7-2 Judgment Results of Positive Reference Substances
[0104]
[0105] Table 8-1 Concentration and Judgment Results of Negative Reference Substances CXCL10 and CCL23
[0106]
[0107]
[0108] Table 8-2 Judgment Results of Negative Reference Substances
[0109]
[0110] According to the test results, by comparing the CXCL10 concentration and the CCL23 concentratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com