Synthesis method and application of 2, 2, 2 (4-bromophenyl)-2-hydroxyacetic acid
A synthesis method and bromophenyl technology are applied in the field of synthesis of bromodifen intermediates, which can solve the problems of low utilization rate of glyoxylic acid, low product yield and the like, and achieve safe and controllable reaction system, high purity of finished products, and high reaction efficiency. mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
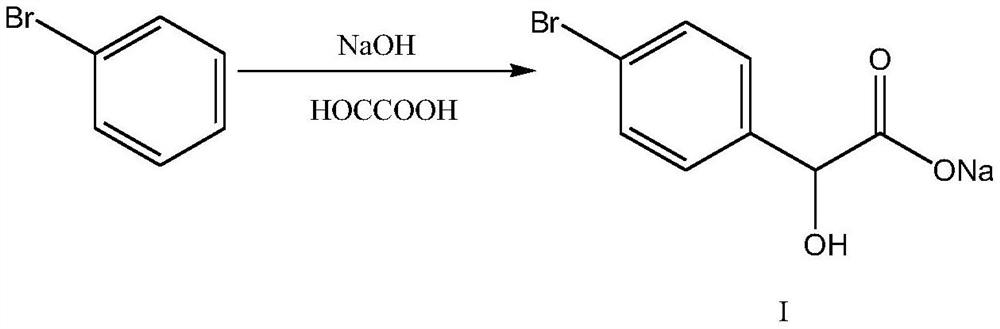
[0051] 1) Add 167g of 30% NaOH to the reaction kettle, add 247g of 30% glyoxylic acid dropwise at -5-0°C, finish the dropwise addition for 1-2h, keep warm for 1-2h, the reaction is over, add 149g of bromobenzene, and heat up the reaction 6-8h to bromobenzene≤1%, the intermediate I was prepared;
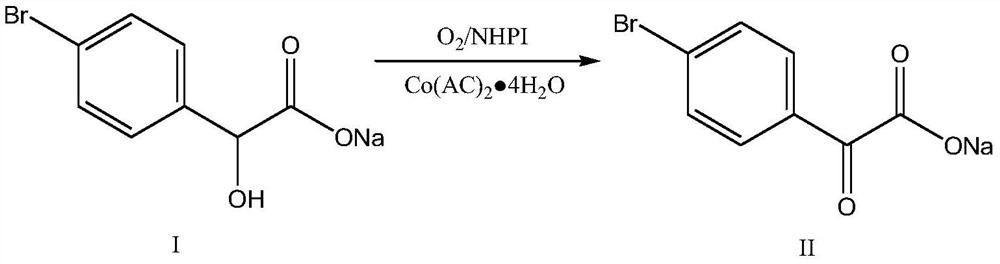
[0052] 2) Intermediate I was adjusted to pH=7-8 with concentrated hydrochloric acid, dehydrated to 120°C under negative pressure, added 100g of toluene, refluxed with water until the water in the kettle was ≤0.1%, removed the toluene under negative pressure, and added 200g of dimethyl sulfoxide , 0.82g N-hydroxyphthalimide, 1.25g cobalt acetate with four crystal waters, at 0.3-0.6MPa, 80-120℃, slowly feed oxygen, and maintain the pressure in the kettle at 0.3-0.6MPa Between, within 4-6h, the pressure in the kettle drops ≤0.01MPa, and the intermediate II is obtained;
[0053] 3) Lower the temperature of intermediate II to 10-20°C, add 165g of bromobenzene and 75.6g of sodium methoxide...
Embodiment 2
[0057] 1) Add 187g of 30% NaOH to the reaction kettle and add 247g of 30% glyoxylic acid dropwise at -5-0°C. After 1-2 hours of dropping, keep warm for 1-2 hours. When the reaction is over, add 152g of bromobenzene and heat up the reaction 6-8h to bromobenzene≤1%, the intermediate I was prepared;
[0058] 2) Intermediate I was adjusted to pH=7-8 with concentrated hydrochloric acid, dehydrated to 120°C under negative pressure, added 100g of toluene, refluxed with water until the water in the kettle was ≤0.1%, removed the toluene under negative pressure, and added quantitatively 200g of dimethylmethylene Sulfone, 1.63g N-hydroxyphthalimide, 2.5g cobalt acetate with four crystal waters, at 0.3-0.6MPa, 80-120°C, slowly feed oxygen, and maintain the pressure in the kettle at 0.3-0.6 Between MPa, within 4-6 hours, the pressure in the kettle drops ≤ 0.01MPa, and intermediate II is produced;
[0059] 3) Lower the temperature of Intermediate II to 10-20°C, add 188.5g of bromobenzene and...
Embodiment 3
[0063] 1) Add 233.5g of 30% KOH to the reaction kettle and add 247g of 30% glyoxylic acid dropwise at -5-0°C. After 1-2 hours of dropping, keep warm for 1-2 hours. When the reaction is over, add 150g of bromobenzene and heat up React for 6-8h until bromobenzene≤1%, and intermediate Ⅰ is obtained;
[0064] 2) Intermediate I was adjusted to pH=7-8 with concentrated hydrochloric acid, dehydrated to 120°C under negative pressure, added 100g of toluene, refluxed with water until the water in the kettle was ≤0.1%, removed the toluene under negative pressure, and added quantitatively 200g of dimethylmethylene Sulfone, 1.1g N-hydroxyphthalimide, 1.5g cobalt acetate with four crystal waters, under the conditions of 0.3-0.6MPa, 80-120℃, slowly feed oxygen, and maintain the pressure in the kettle at 0.3-0.6 Between MPa, within 4-6 hours, the pressure in the kettle drops ≤ 0.01MPa, and intermediate II is produced;
[0065] 3) Lower the temperature of Intermediate II to 10-20°C, add 170.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com