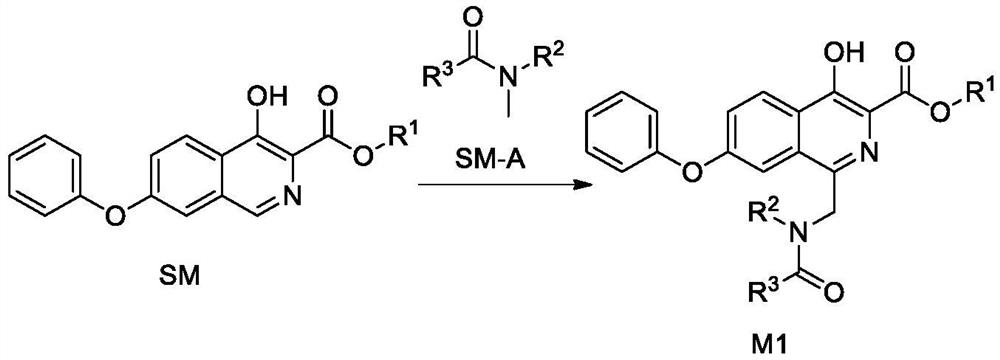
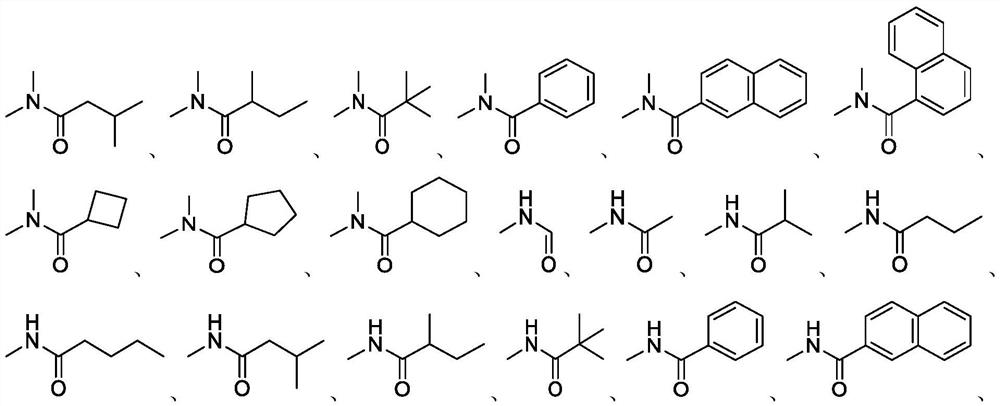
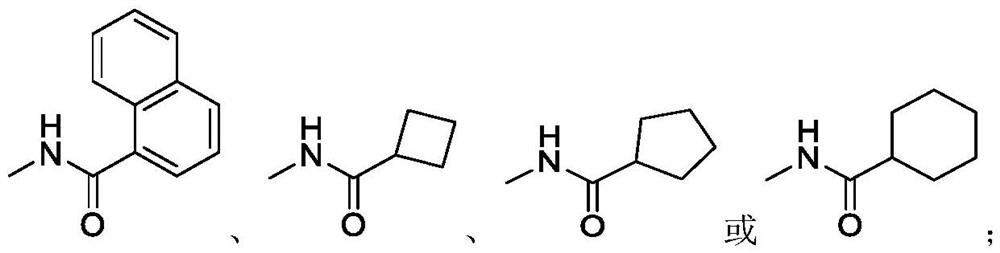
Synthetic method of roxadustat and intermediate thereof, and intermediate of roxadustat
A synthetic method and compound technology, applied in the direction of organic chemistry, can solve the problems of unfavorable industrial production, cumbersome operation, long steps, etc., and achieve the effect of less three wastes, simple post-processing, and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0209] The specific embodiment of the preparation method of roxadustat of the present invention and the synthesis method of its intermediate are described in the following examples:
[0210] In the following examples, where the temperature is not specified for reactions or operations, etc., it generally means that the temperature is carried out at room temperature.
[0211] In the following examples, unless otherwise stated, the purity refers to HPLC purity or GC purity.
Embodiment 1
[0214] The synthesis of embodiment 1 roxadustat
[0215] The synthetic route is as follows:
[0216]
[0217] 1.1 Synthesis of Intermediate M1-1
[0218] Heat 2.95g (0.01mol) of compound 5 in 29.5g (about 0.4mol) of N,N-dimethylformamide (DMF) to 60°C-70°C to dissolve completely, and use 4.76g (0.02mol) of sodium persulfate 9.6 g (about 0.53 mol) of water was dissolved and added to the above solution, and stirred for 6 hours. TLC monitors that the reaction is complete, the reaction solution is cooled to room temperature, ethyl acetate (80mL) is added, washed with water (40mL×4), the organic phase is concentrated to a volume of 8-10mL, suction filtered, washed with a small amount of ethyl acetate, and the filter cake is vacuum-dried , 2.7 g of white solid was obtained, the yield was 73.5%, and the purity was 97.22%.
[0219] MS(ESI) m / z: 367.1[M+1] + , 1 H-NMR (400MHz, DMSO-d 6)δ11.62(s,1H),8.38(t,J=8.1Hz,1H),8.11(d,J=58.9Hz,1H),7.72(d,J=22.0Hz,1H),7.44-7.58( m,3H),7....
Embodiment 2
[0235] The synthesis of embodiment 2 intermediate 9
[0236] The synthetic route is as follows:
[0237]
[0238] 2.1 Synthesis of Intermediate M1-2
[0239] Heat 2.95g (0.01mol) of compound 5 in 29.5g (about 0.4mol) of N-methylacetamide to 60°C-70°C to completely dissolve, and 4.76g (0.02mol) of sodium persulfate with 9.6g (about 0.53mol) ) was dissolved in water and added to the above solution, and stirred for 8 hours. TLC monitors that the reaction is complete, the reaction solution is cooled to room temperature, ethyl acetate (80mL) is added, washed with water (30mL×4), the organic phase is concentrated to a volume of about 8-10mL, suction filtered, washed with a small amount of ethyl acetate, and dried. 2.4 g of white solid was obtained, the yield was 65.0%, and the purity was 98.76%.
[0240] MS(ESI) m / z: 367.2[M+1] + , 1 H-NMR (400MHz, DMSO-d 6 )δ11.59(s,1H),8.36(t,J=7.3Hz,2H),7.71(s,1H),7.56(d,J=9.0Hz,1H),7.46(t,J=7.4Hz, 2H), 7.25(t, J=7.3Hz, 1H), 7.14(d, J=7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com