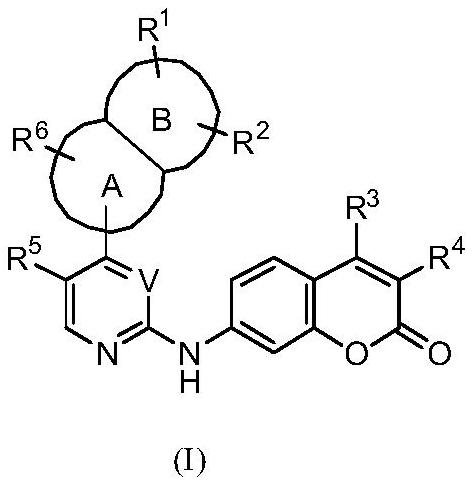
Protein kinase inhibitor and derivatives thereof, preparation method, pharmaceutical composition and application
A technology of protein kinase inhibitors and derivatives, which is applied in the field of protein kinase inhibitors and derivatives, can solve the problems of cell cycle regulation and transcription abnormality, and achieve the effects of simple preparation method, excellent therapeutic effect and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
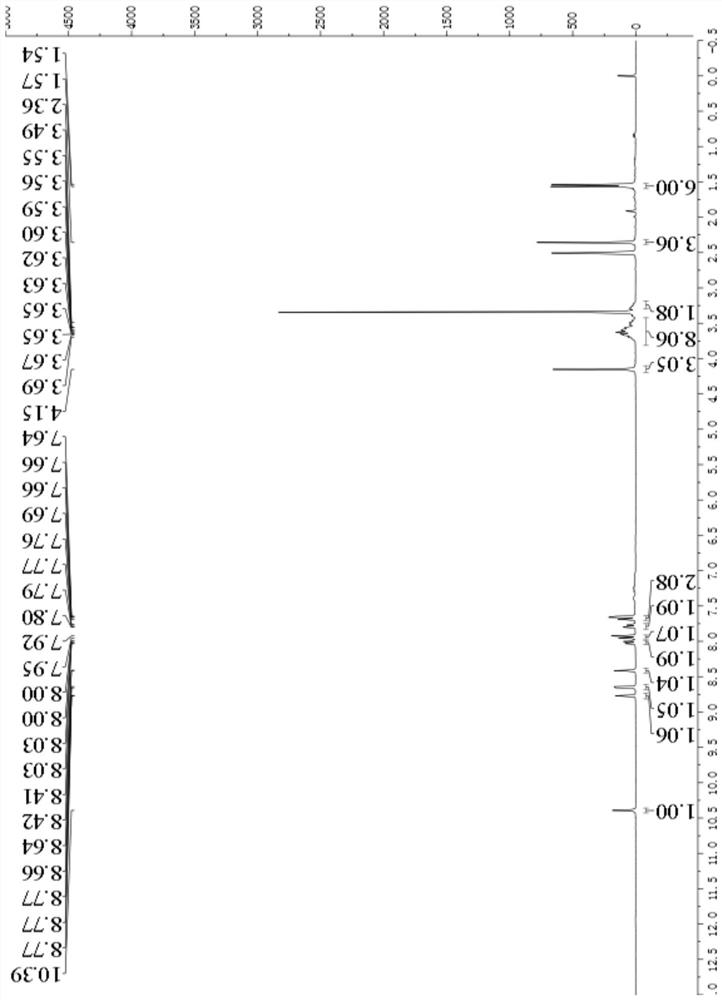
[0108] Example 1: 3-acetyl-7-((4-(benzofuran-7-yl)pyrimidin-2-yl)amino)-4-morpholinyl-2H-benzopyran-2-one ( Synthesis of compound I-1)
[0109]
[0110] (1) Synthesis of benzofuran-7-boronic acid pinacol ester (compound 1a)
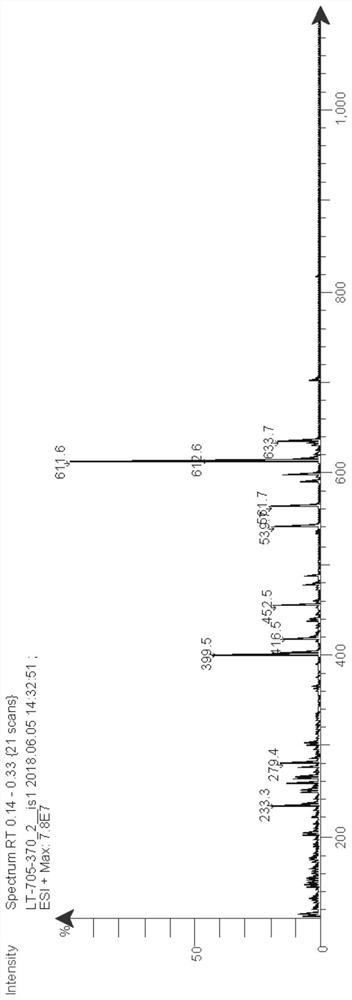
[0111]Add 7-bromobenzofuran (394mg, 2mmol), pinacol diborate (762mg, 3mmol), [1,1'-bis(diphenylphosphino)ferrocene] to a 25mL two-necked flask Palladium chloride (146mg, 0.2mmol), sodium carbonate (424mg, 4mmol), 2mL of water and 12mL of 1,4-dioxane, under nitrogen protection, react at 100°C for 12h, after the reaction, add 50mL of water and 100 mL of ethyl acetate, the organic layer was collected, concentrated and purified by column chromatography to obtain 346 mg of a colorless oil, with a yield of 71%. ESI-MS m / z:245[M+H] + .
[0112] (2) Synthesis of 4-(benzofuran-7-yl)pyrimidin-2-amine (compound 1-1)
[0113] Add compound 1a (293mg, 1.20mmol), 2-amino-4-chloropyrimidine (130mg, 1.00mmol), [1,1'-bis(diphenylphosphino)ferrocene] di Palladium c...
Embodiment 2
[0131] Example 2: 7-((4-(1H-indol-1-yl)pyrimidin-2-yl)amino)-3-acetyl-4-morpholinyl-2H-benzopyran-2-one Synthesis of (I-21)
[0132]
[0133] (1) Synthesis of 4-((1H-indol-1-yl)pyrimidin-2-amine (compound 3-1)
[0134] In a 50mL single-necked bottle, add indole (702mg, 6mmol), 2-amino-4-chloropyrimidine (650mg, 5mmol), cesium carbonate (3.26g, 10mmol) and 15mL DMF, react overnight at 100°C, After the reaction, add 100mL of water, extract with 3×50mL of ethyl acetate, collect the organic layer, concentrate and separate and purify by column chromatography (petroleum ether:ethyl acetate=3:1) to obtain 370mg of white solid, yield 35% . ESI-MS m / z:211[M+H] + .
[0135] (2) 7-((4-(1H-indol-1-yl)pyrimidin-2-yl)amino)-3-acetyl-4-morpholinyl-2H-benzopyran-2-one ( Compound I-21) Synthesis
[0136] Using compound 3-1 (126 mg, 0.6 mmol) and compound 2-1 (278 mg, 0.66 mmol) as raw materials, referring to the preparation method of compound I-1, 173 mg of compound I-21 was obtained ...
Embodiment 3
[0140] Example 3: 3-acetyl-7-((4-(3-isopropyl-2-methyl-2H-indazol-5-yl)pyrimidin-2-yl)amino)-4-morpholinyl Synthesis of -2H-benzopyran-2-one (I-29)
[0141]
[0142] (1) Synthesis of 5-bromo-2-methyl-2H-indazole (compound 4a)
[0143] Add 5-bromoindazole (9.95g, 50mmol) into a 250mL single-necked flask, add 150mL DMF to dissolve, slowly add sodium hydride (1.44g, 60mmol) under ice-cooling, add iodomethane (8.52g, 60mmol) after stirring for 30min , after reacting at room temperature for 5 h, the reaction was quenched by adding a saturated solution of sodium thiosulfate, extracted with ethyl acetate and concentrated, and the residue was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 5: 1) to obtain light yellow Solid 4.1g, yield 32.4%. 1 H-NMR (300MHz, DMSO-d 6 )δ8.34(s,1H),7.92-7.98(m,1H),7.57(dd,J=9.1,0.8Hz,1H),7.30(dd,J=9.1,1.9Hz,1H),4.17(s ,3H).
[0144] (2) Synthesis of 2-(5-bromo-2-methyl-2H-indazol-3-yl)propan-2-ol (compound 4b)
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com