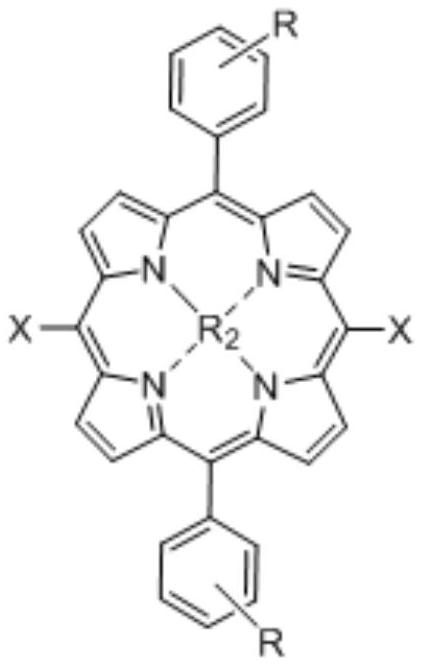
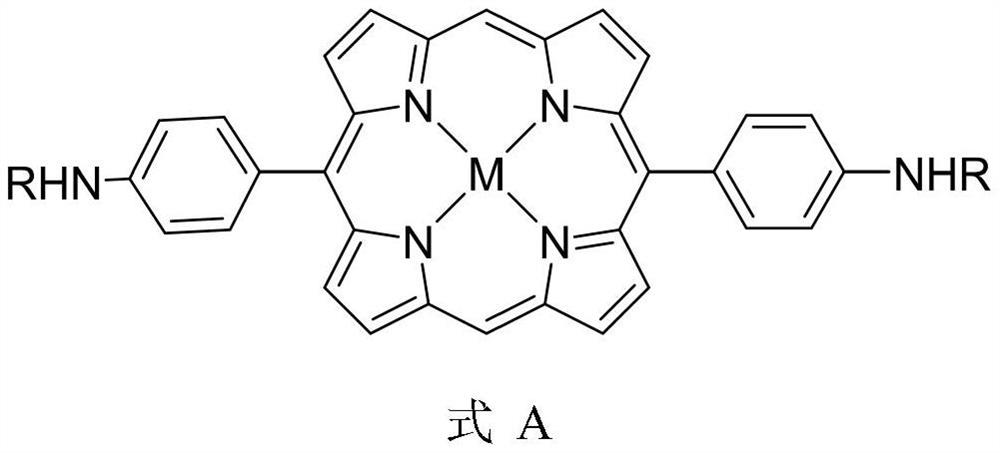
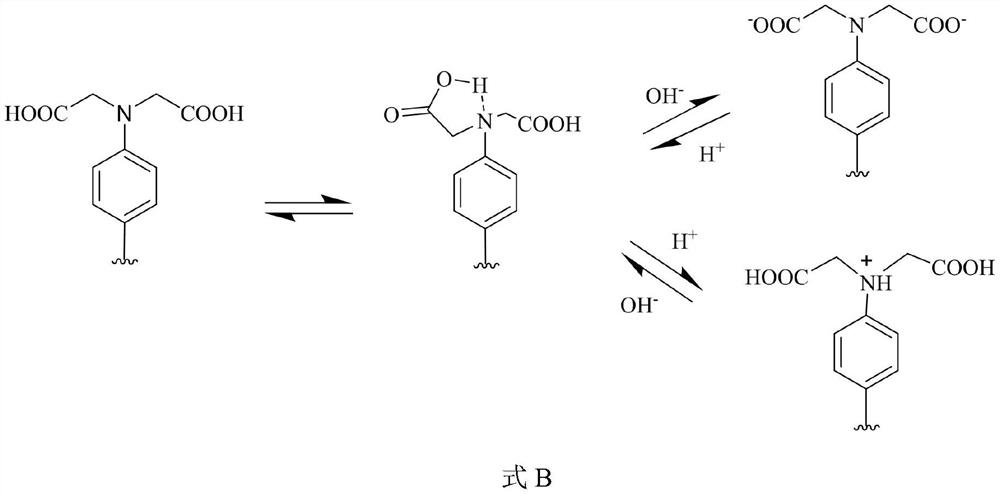
Novel tetrapyrrole compound and application thereof
A tetrapyrrole compound and compound technology, applied in the field of photosensitive drugs and photodynamic therapy, can solve the problems of unstable structure, strong skin phototoxicity, and high cost, and achieve the effect of low skin phototoxic side effects and significant photodynamic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] 5,15-bis[4-(N,N-dicarboxymethyl)aminophenyl]porphine (I 1 ) preparation method
[0076]
[0077] Compound I 1a (2.7mmol) and dipyrromethane (0.395g, 2.7mmol) were dissolved in DCM (500mL), and trifluoroacetic acid (0.12mL, 1.7mmol) was added dropwise under nitrogen protection, and the reaction was stirred at room temperature for 3h. Dichlorodicyanobenzoquinone DDQ (0.735 g, 3.24 mmol) and triethylamine (4 mL) were added, and the stirring reaction was continued for 3 h. The solvent was distilled off under reduced pressure, and the obtained residue was separated and purified by column chromatography (MeOH / DCM=1 / 200) to obtain a purple solid I 1b (1.2mmol), yield 44.3%. 1 HNMR (400MHz, CDCl 3 ): δppm 10.31(s, 2H), 9.41(d, J=3.7Hz, 4H), 9.18(d, J=3.5Hz, 4H), 8.17(d, J=8.4Hz, 4H), 7.08(d, J=8.8Hz, 4H), 4.48(s, 8H), 4.41(q, J=7.3Hz, 8H), 1.44(t, J=7.2Hz, 12H), -3.02(s, 2H).MS(MALDI -TOF)m / z[M+H] + ,837.3.
[0078] Compound I 1b (0.50mmol) dissolved in THF / MeOH (50...
Embodiment 2
[0080] 5,15-bis[4-(N,N-dicarboxyethyl)aminophenyl]porphine (I 2 ) preparation method
[0081]
[0082] Compound I 1 Similar preparation method, obtained compound I 2 , yield 31.8%. 1 H NMR (400MHz, DMSO-d 6 ): δppm 12.27(s, 4H), 10.46(s, 2H), 9.56(d, J=4.2Hz, 4H), 9.20(d, J=4.4Hz, 4H), 8.16(d, J=8.0Hz, 4H), 7.35(d, J=8.0Hz, 4H), 3.62(d, J=8.6Hz, 8H), 2.60(t, J=7.3Hz, 8H), -2.87(s, 2H). HRMS(MALDI):m / z calcd for C 44 h 41 N 6 o 8 [M+H] + , 781.2902; found, 781.2805.
Embodiment 3
[0084] 5,15-bis[4-(N,N-dicarboxy-n-propyl)aminophenyl]porphine (I 3 ) preparation method
[0085]
[0086] Compound I 1 Similar preparation method, obtained compound I 3 , yield 29.8%. 1 H NMR (400MHz, DMSO-d 6 ): δppm 12.25(s, 4H), 10.55(s, 2H), 9.61(d, J=4.4Hz, 4H), 9.16(d, J=4.4Hz, 4H), 8.10(d, J=8.0Hz, 4H), 7.27(d, J=8.0Hz, 4H), 3.59(d, J=9.0Hz, 8H), 2.48(t, J=7.2Hz, 8H), 2.46-2.00(m, 8H), -2.99 (s,2H). 13 C NMR (100MHz, DMSO-d 6 ): δppm 174.91, 147.60, 144.69, 136.66, 132.66, 131.33, 111.13, 105.86, 50.15, 31.50, 22.82. HRMS (MALDI): m / z calcdfor C 48 h 49 N 6 o 8 [M+H] + , 837.3506; found, 837.3569.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com