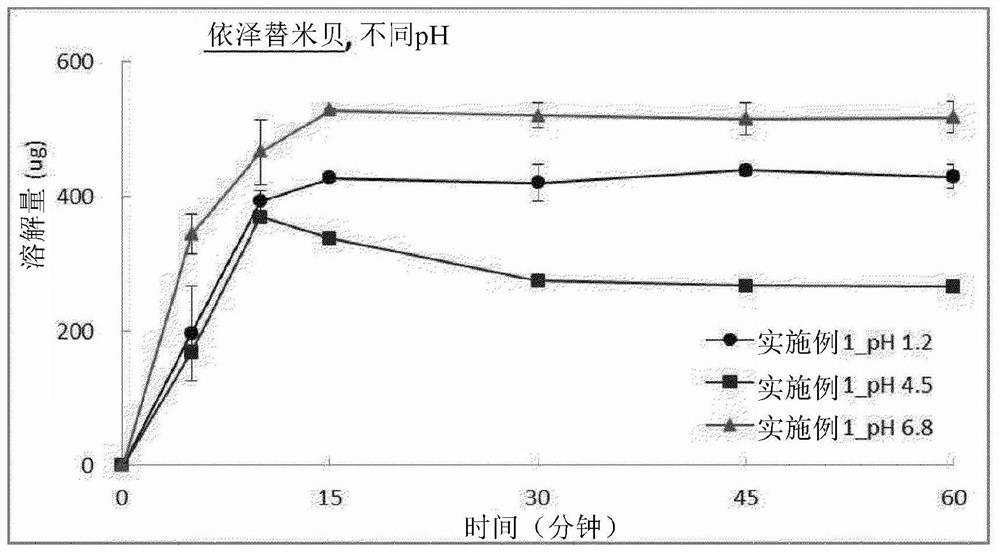
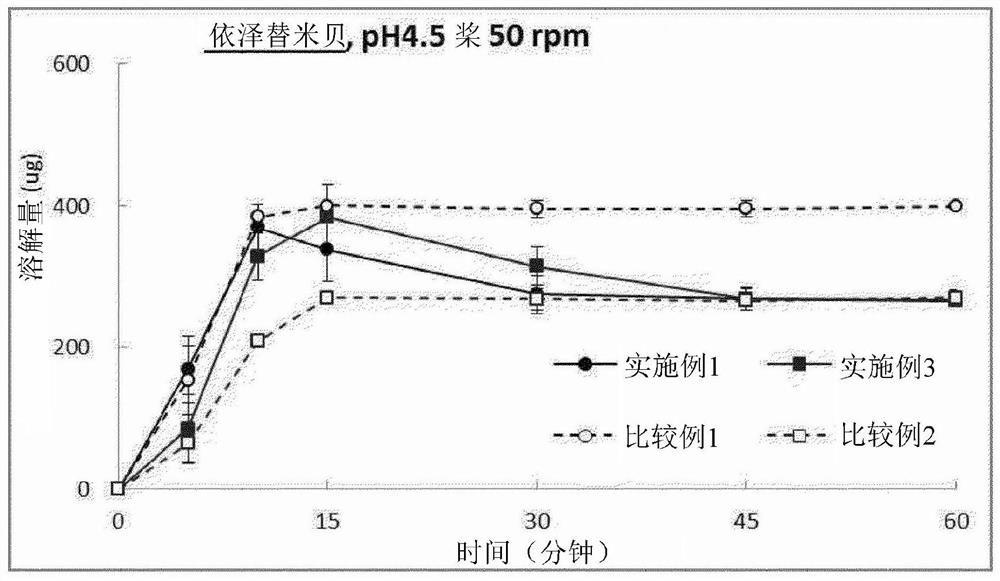
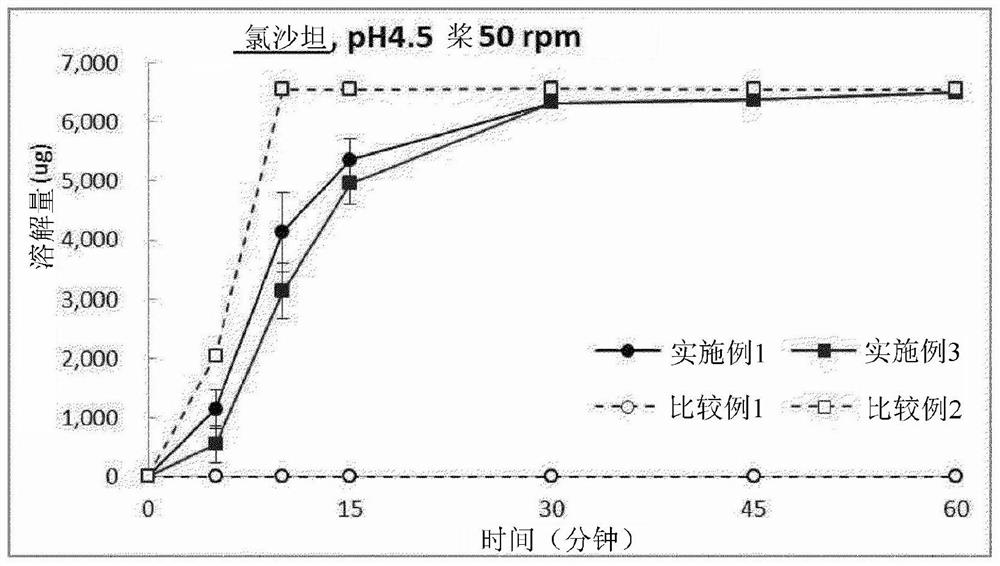
Pharmaceutical combination preparation comprising ezetimibe and losartan
A technology of ezetimibe and losartan, applied in the field of drug combination preparations, can solve the problems of reduced productivity and low particle fluidity, and achieve the effect of ensuring biological equivalence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Preparation of a bilayer tablet comprising ezetimibe and losartan
[0060] Coated bilayer tablets containing ezetimibe and losartan as active ingredients were prepared according to the composition shown in Table 1 below.
[0061] Specifically, ezetimibe was placed in a fluidized bed granulator together with lactose hydrate, microcrystalline cellulose, croscarmellose sodium and sodium lauryl sulfate and mixed for 3 minutes. To this was added a binder solution in which povidone was dissolved in water and the delivery pump was set to 2 rpm and combined for about 50 minutes of granulation. The resulting product was dried in a fluidized bed dryer at 45° C. and then sieved through a 0.6 mm mesh size to yield ezetimibe granules. At this time, the content of particles with a diameter greater than 250 μm in the ezetimibe particles was 5% by weight.
[0062] Losartan potassium was placed in a mixer with lactose hydrate, microcrystalline cellulose and crospovidone and...
Embodiment 2
[0065] Example 2: Preparation of a bilayer tablet comprising ezetimibe and losartan
[0066] A coated bilayer tablet containing ezetimibe and losartan as active ingredients was prepared in the same manner as in Example 1 according to the composition shown in Table 1 below.
Embodiment 3
[0067] Example 3: Preparation of capsules containing microtablets comprising ezetimibe and losartan
[0068] Capsules containing coated microtablets each containing ezetimibe and losartan as active ingredients were prepared according to the composition shown in Table 1 below.
[0069] Specifically, ezetimibe granules and losartan granules were prepared in the same manner as in Example 1. Microtablets containing losartan (303 mg / 30T) and microtablets containing ezetimibe (157 mg / 10T) (which are 2 mm in diameter and have a hardness of about 1.5 kp ). Microtablets containing losartan and microtablets containing ezetimibe were coated separately using a fluidized bed coater according to the coating formulation shown in Table 1, and filled to size 0 In hard capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com