Method for preparing cyclothiourea compound
A technology of urea compound and cyclic thiourea, applied in organic chemistry and other fields, can solve problems such as metal waste pollution, large catalyst loading, and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
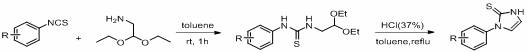
[0026] Synthetic example The preparation of raw material 9x-9ae
[0027]
[0028] Taking R as hydrogen to prepare raw material 9x as an example, in a two-neck flask, phenylisothiocyanate (4.0 mmol, 1.0 equiv) and aminoacetaldehyde diethyl acetal (4.0 mmol, 1.0 equiv) were stirred at room temperature for 1 h, TLC monitors that the reaction of the phenylisothiocyanate raw material is complete. A catalytic amount of 37% concentrated hydrochloric acid (0.4 mL, 10 mol%) was added to the reaction solution, stirred at room temperature for 5 min, then moved to an oil bath at 110 °C and heated to reflux. The reaction progress was monitored by TLC, and the reaction was completed after 3 h. After the reaction solution was cooled to room temperature, the toluene solvent in the reaction solution was spin-dried, washed with 1N NaOH aqueous solution, extracted 5 times with ethyl acetate, combined the organic layers, washed with saturated sodium chloride solution, dried over anhydrous sodi...
Embodiment 1
[0032]
[0033]Weigh NaH (1.2 mmol, 4.0 equiv) in a reaction flask, suspend in anhydrous THF (0.8 mL) and stir, add cyclic thiourea 9 (0.3 mmol, 1.0 equiv, dissolved in 0.2 mL DMA ), stirred at room temperature for 2 min after the addition, then added diiodobenzene 2a (0.6 mmol, 2.0 equiv, dissolved in 0.2 mL THF), continued to stir at room temperature, and TLC monitored the completion of the reaction. After the reaction is complete, add ice water and tetrahydrofuran to quench the reaction, extract three times with ethyl acetate, combine the organic layers, wash with saturated sodium chloride solution, dry with anhydrous sodium sulfate, filter, spin to dry the solvent, add appropriate amount of silica gel powder to mix the sample , separated by flash column chromatography to obtain the product cyclic thiourea compound 10.
[0034] When R is hydrogen, the reaction ends in 3 hours, and the yield is 70%; when R is 4-Cl, the reaction ends in 2 hours, and the yield is 88%; when ...
Embodiment 2
[0036]
[0037] Weigh NaH (1.2 mmol, 4.0 equiv) in a reaction flask, suspend in anhydrous THF (0.8 mL) and stir, add cyclic thiourea 9ae (0.3 mmol, 1.0 equiv, dissolved in 0.2 mL DMA) dropwise during stirring ), stirred at room temperature for 1.5 min after the addition, then added diiodobenzene 2a (0.6 mmol, 2.0 equiv, dissolved in 0.2 mL THF), continued to stir at room temperature, and TLC monitored the completion of the reaction. After 4 hours of reaction, add ice water and tetrahydrofuran to quench the reaction, extract 3 times with ethyl acetate, combine the organic layers, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter, spin to dry the solvent, and add appropriate amount of silica gel powder The sample was mixed and separated by flash column chromatography to obtain the product cyclic thiourea compound 10ae with a yield of 60%.
[0038] The nuclear magnetic data of above-mentioned product cyclic thiourea compound is as follows:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com