Engineered cells and uses thereof
A cell, immune cell technology, applied in the field of engineered cells and their uses, can solve problems such as off-target toxicity, release syndrome, and unwanted cytokines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0193] Various aspects of the present disclosure are further illustrated by the following non-limiting examples.
example 1
[0194] Example 1: Design of a TCR+ CAR construct containing only a co-stimulatory domain
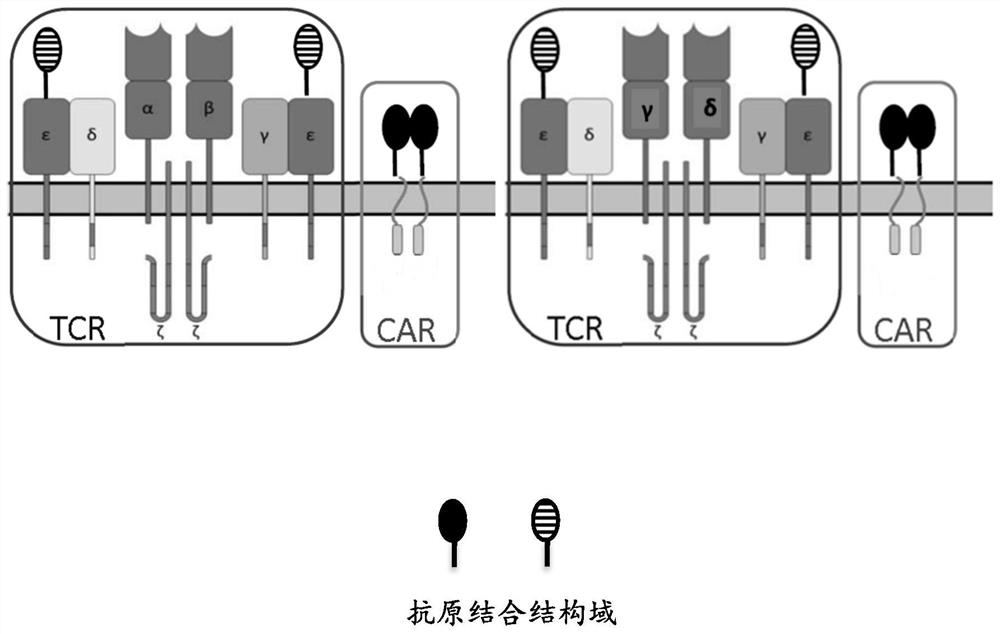
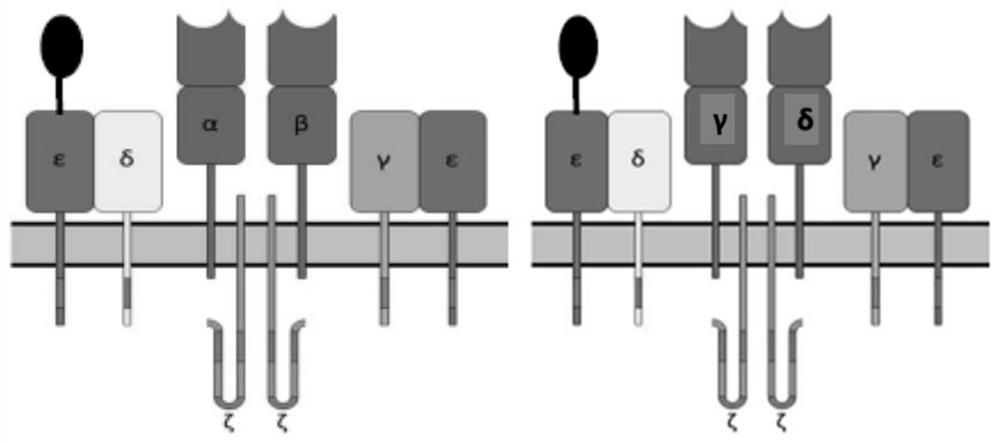
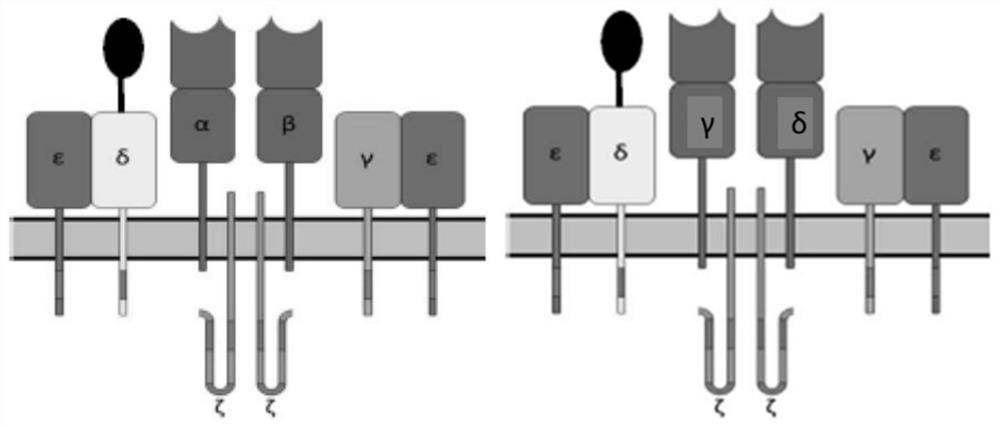
[0195] This example describes the design of an exemplary TCR+ CAR construct comprising only a co-stimulatory domain. Twelve constructs were designed, each comprising a polynucleotide as follows:
[0196] Construct 1 (anti-CLL-1 TCRε+anti-CD33 co-stimulatory CAR): polynucleotide contains CD3ε leader peptide from N-terminus to C-terminus-anti-CLL-1 sdAb-(G4S) 3 -CD3ε-T2A-CD8 leader peptide-anti-CD33 sdAb-CD8 hinge-CD8 transmembrane-CD27 co-stimulatory domain;
[0197] Construct 2 (anti-CLL-1 TCRδ+anti-CD33 co-stimulatory CAR): polynucleotide contains CD3δ leader peptide from N-terminus to C-terminus-anti-CLL-1 sdAb-(G4S) 3 -CD3δ-T2A-CD8 leader peptide-anti-CD33 sdAb-CD8 hinge-CD8 transmembrane-CD27 co-stimulatory domain;
[0198] Construct 3 (anti-CLL-1 TCRγ + anti-CD33 co-stimulatory CAR): polynucleotide contains CD3γ leader peptide from N-terminus to C-terminus-anti-CLL-1 sdAb-(G4S) ...
example 2
[0222] Example 2: Viral transfection and virion production
[0223]To generate viral particles comprising polynucleic acids encoding any of the systems disclosed herein, a mixture of lentiviral packaging plasmids including pMDLg / pRRE (Addgene #12251), pRSV-Rev (Addgene #12253) and pMD2.G (Addgene #12259) was mixed with PLVX-EF1A (including target system) vector was premixed with polyetherimide (PEI) at a pre-optimized ratio, mixed well and incubated at room temperature for 5 minutes. Add the transfection mixture dropwise to 293-T cells and mix gently. Incubate the transfected 293-T cells at 37°C and 5% CO 2 Incubate overnight. Twenty-four hours after transfection, the supernatant was collected and centrifuged at 500 g for 10 min at 4°C to remove any cell debris. The centrifuged supernatant was filtered through a 0.45 μm PES filter, and the virus supernatant was concentrated after ultracentrifugation. After centrifugation, carefully discard the supernatant and wash the virus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com