Primer, probe and kit for quantitatively detecting BCR-ABL1 fusion gene
A technology of BCR-ABL1 and BCR-BAL1, which is applied in the field of probes and kits, and primers for quantitative detection of BCR-ABL1 fusion genes, which can solve the problems of cumbersome operation, limited throughput, and high cost of digital PCR systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Embodiment 1: kit and detection method
[0114] This embodiment provides the composition, packaging and quantity (20 reactions / box) of a kit for quantitative detection of BCR-ABL1 fusion gene, as shown in Table 8.
[0115] Table 8. The composition, packaging and quantity of the kit
[0116]
[0117]
[0118] This example provides a method for quantitative detection of the BCR-ABL1 fusion gene using the above kit. The specific implementation steps are as follows:
[0119] Step 1: RNA template extraction and quality inspection of samples to be tested
[0120] 1) Take 2mL of peripheral blood from leukemia patients and place it in blood collection tubes with EDTA or sodium citrate anticoagulant, mark it to ensure that the label information is correct, and store it at 4°C.
[0121] 2) Use a nucleic acid extraction or purification kit (Yue Sui Machinery No. 20170583) for nucleic acid extraction.
[0122] 3) Use a spectrophotometer (such as NanoDrop2000 ultra-micro s...
Embodiment 2
[0161] Example 2: Sensitivity detection and minimum detection rate experiment of the kit
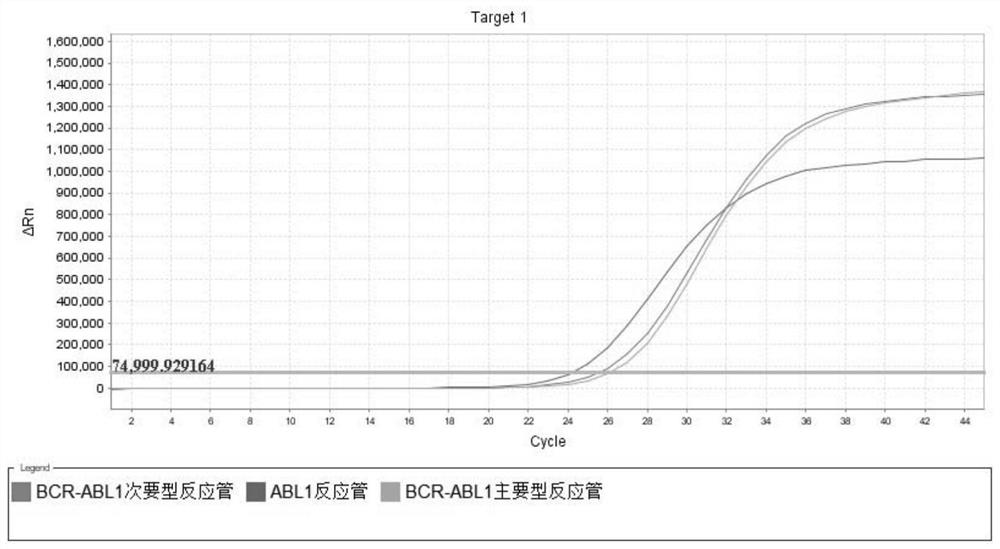
[0162] BCR-ABL1 major type sample L1 and BCR-ABL1 minor type sample L2 with a calibrated concentration close to the detection limit of 500 copies / mL were selected for verification. Follow step 1 to extract nucleic acid, step 2 to test the sample, and do 10 multiple tubes for each test. The operation was carried out strictly according to the instructions of the kit, and the detection was performed on a real-time PCR system. After the amplification is completed, read and analyze the experimental results according to step 3, and judge the results in step 4.
[0163] Sensitivity and the minimum detection limit of the present invention are detected, and the test results are shown in Table 12 below.
[0164] Table 12 The results of the minimum detection limit of the kit
[0165]
[0166] The sensitivity detection result of the kit is consistent with the theoretical value, and the sensiti...
Embodiment 3
[0167] Embodiment 3: the repeatability of kit
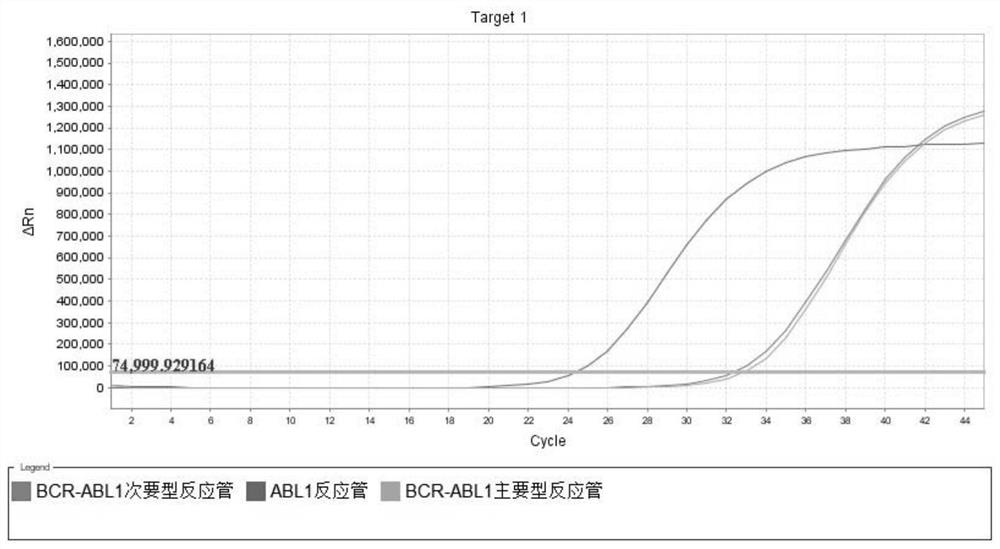
[0168] According to the measured copy number of the control sample, a total of 4 replicate reference products were prepared, numbered R1-R4 respectively. R1-R2 is composed of BCR-ABL1 major type (P210) positive sample nucleic acid mixed with 10ng / μL negative sample nucleic acid; R3-R4 is composed of BCR-ABL1 minor type (P190) positive sample nucleic acid mixed with 10ng / μL negative sample nucleic acid made. Nucleic acid samples of BCR-ABL1 major type (P210) and BCR-ABL1 minor type (P190) were confirmed by digital PCR for copy number confirmation.
[0169] Using the prepared R1-R4 as the samples to be tested, test the repeatable reference product (R1-R4), repeat the test 10 times, and perform statistical calculations on the coefficient of variation of the logarithmic value of the sample concentration for 10 times. R1-R2 test results were all positive for BCR-ABL1 major type (P210) fusion gene; R3-R4 test results were all positiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com