Diazabenzofluoranthene compound as well as preparation method and application thereof
A technology of diazofluoranthene and benzofluoranthene, which is applied in the field of diazofluoranthene compounds and their preparation, can solve problems such as narrow spectral range, achieve wide spectral range, good application prospects, and significant economical value effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
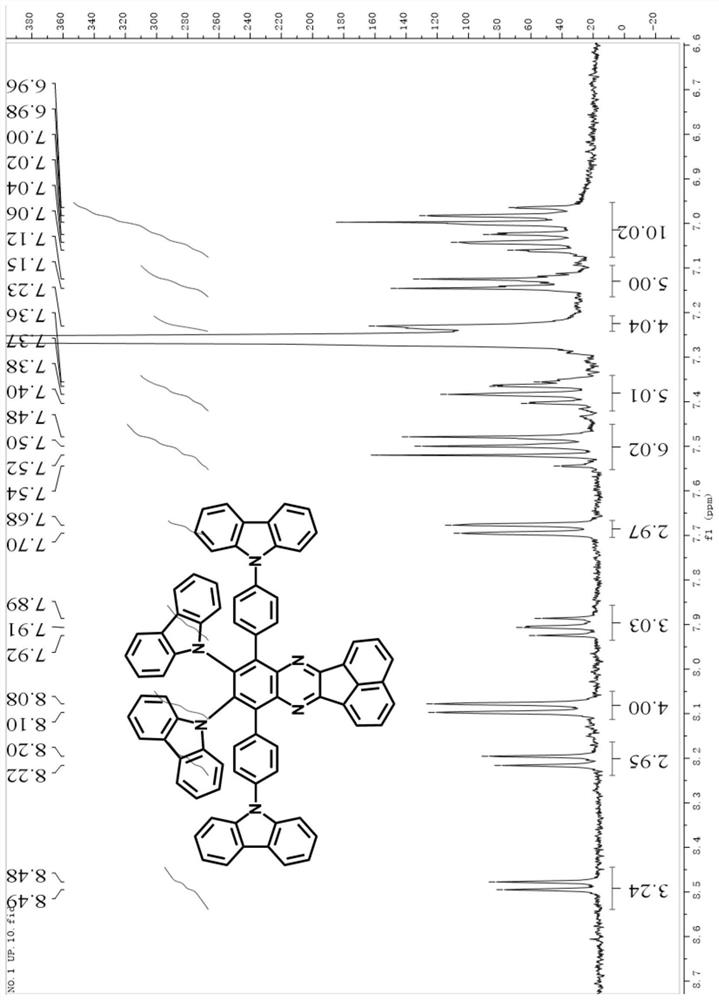
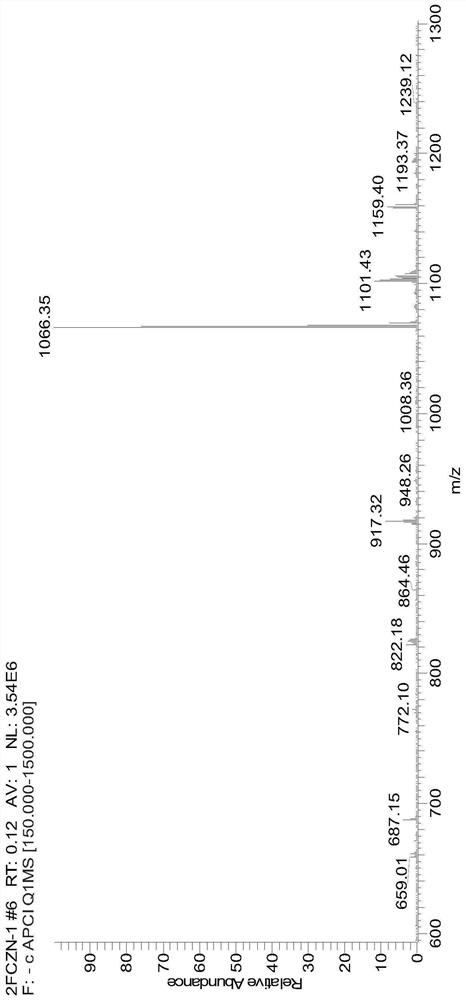
[0055] A diazobenzofluoranthene compound, its structural formula is as follows:
[0056]
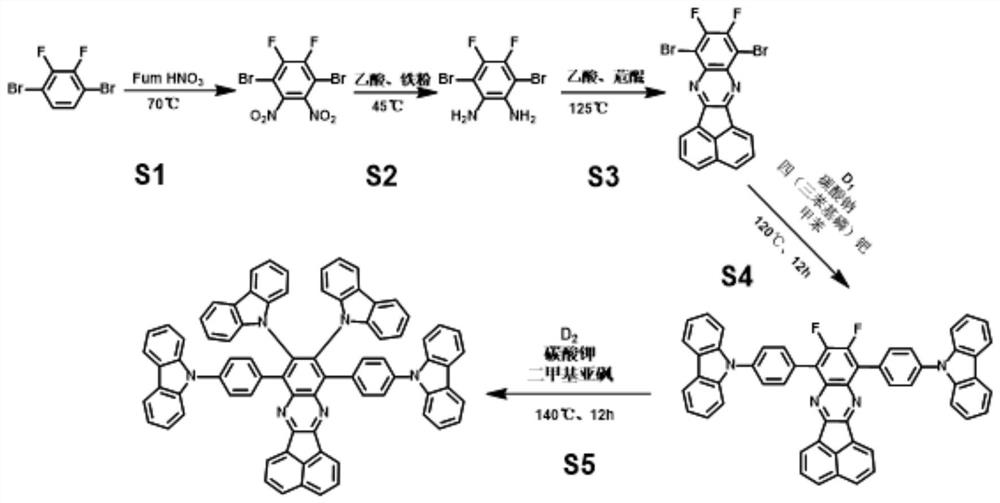
[0057] The synthetic route of this compound is as follows figure 1 Shown, its preparation method comprises the steps:
[0058] Preparation of S1.1,4-dibromobenzene-2,3-difluoro-5,6-dinitrobenzene:
[0059] Put the three-necked flask (250mL), spherical condenser, atmospheric dropping funnel, glass stopper and stirring bar into an oven, and dry at 100°C for 30min. Take out the dried three-necked flask, add 50mL trifluoromethanesulfonic acid dropwise, then slowly add 2.5mL fuming nitric acid, stir in ice bath for 30min; then, add 18.4mmol of 1,4-dibromo-2 , 5g of 3-difluorobenzene, stirred at room temperature for 2h, cooled the mixture to 0°C, then slowly added 2.5mL of fuming nitric acid, heated to 70°C and reacted for 30h; finally, added sodium hydroxide under ice bath conditions to adjust the pH The value was adjusted to 7, filtered and collected to obtain a light yellow solid with...
Embodiment 2
[0074] A preparation method of a diazobenzofluoranthene compound, comprising the steps of:
[0075] In step S1, the molar ratio of 1,4-dibromo-2,3-difluorobenzene, fuming nitric acid and trifluoromethanesulfonic acid is 1:9:70, the nitration reaction temperature is 70°C, and the reaction time is 24h, the product Rate is 44%, and others are with embodiment 1.
[0076] In step S2, the molar ratio of 1,4-dibromobenzene-2,3-difluoro-5,6-dinitrobenzene, iron powder and acetic acid is 1:15:175, the reduction reaction temperature is 40°C, and the reaction time is Be 8h, productive rate is 70%, other is with embodiment 1.
[0077] In step S3, the molar ratio of 3,6-dibromobenzene-4,5-difluoro-1,2-phenylenediamine to acenaphthenequinone compound is 1:1, the condensation reaction temperature is 100°C, and the reaction time is 16h. Productive rate is 70%, other is with embodiment 1.
[0078] Intermediate α, basic salt, palladium-containing coupling agent and electron donor group D in ...
Embodiment 3
[0081] A preparation method of a diazobenzofluoranthene compound, comprising the steps of:
[0082] In step S1, the molar ratio of 1,4-dibromo-2,3-difluorobenzene, fuming nitric acid and trifluoromethanesulfonic acid is 1:8:45, the nitration reaction temperature is 50°C, and the reaction time is 36h, the product Rate is 72%, and others are with embodiment 1.
[0083] In step S2, the molar ratio of 1,4-dibromobenzene-2,3-difluoro-5,6-dinitrobenzene, iron powder and acetic acid is 1:20:150, the reduction reaction temperature is 60°C, and the reaction time is Be 6h, productive rate is 80%, other is with embodiment 1.
[0084] In step S3, the molar ratio of 3,6-dibromobenzene-4,5-difluoro-1,2-phenylenediamine to acenaphthenequinone compound is 1:1, the condensation reaction temperature is 125°C, and the reaction time is 8h. Productive rate is 75%, other is with embodiment 1.
[0085] Intermediate α, basic salt, palladium-containing coupling agent and electron donor group D in s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com