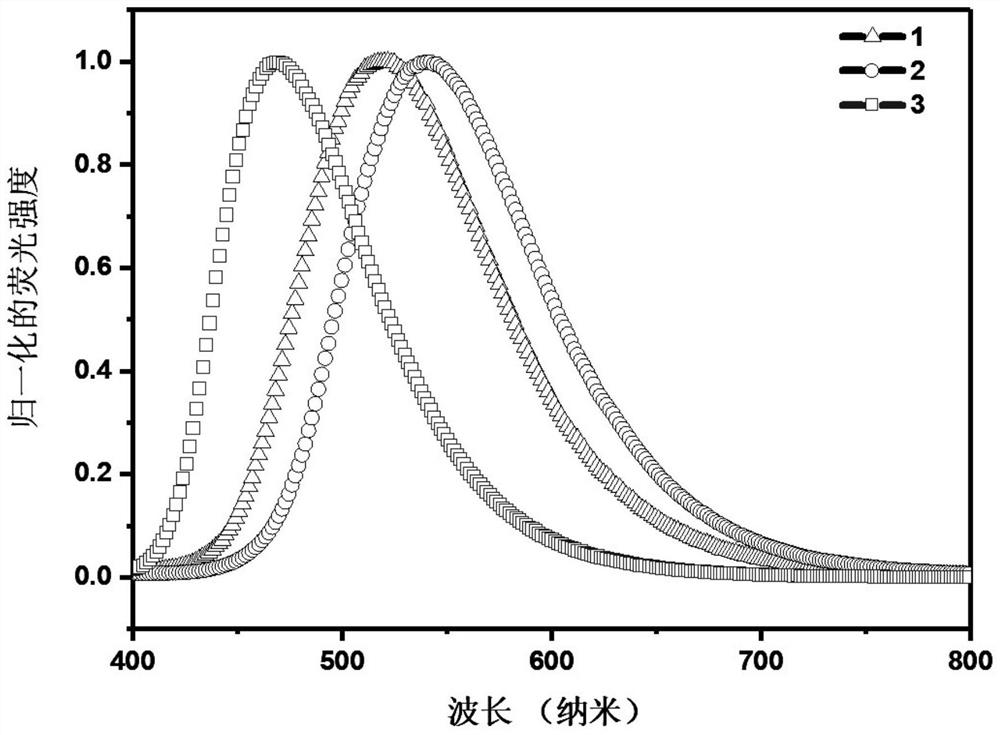
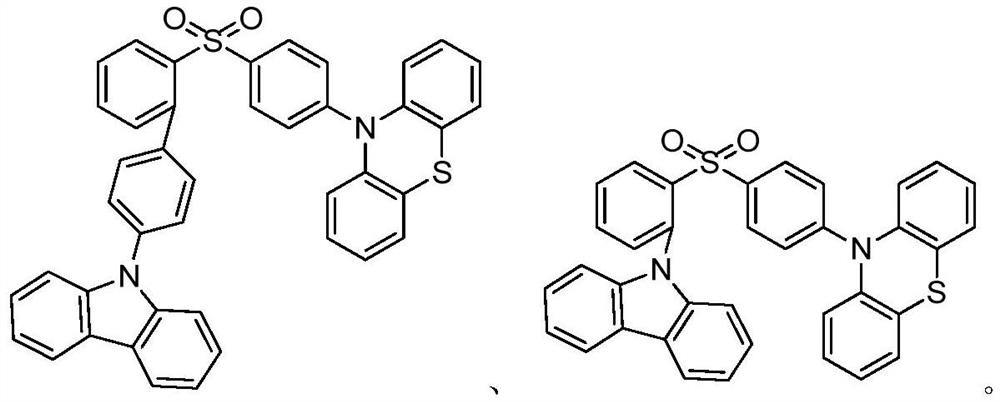
Tadf material based on large steric hindrance space charge transfer and its synthesis method and application
A technology of space charge and large steric resistance, which is applied in the direction of luminescent materials, chemical instruments and methods, circuits, etc., can solve the problems of high preparation cost, high price, and efficiency roll-off, and achieve simple synthesis process, adjustable luminous color, The effect of high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The above-mentioned synthetic method based on the large steric hindrance space charge transfer TADF material is characterized in comprising the following steps:
[0033] method one:
[0034] (1) 2-bromobenzenesulfonyl chloride, 2-fluorobenzenesulfonyl chloride, 2-bromobenzoyl chloride or 2-fluorobenzoyl chloride and fluorobenzene or bromobenzene are obtained by Friedel-Crafts reaction of 2-substituted fluoro or Brominated intermediates;
[0035] (2) The intermediate obtained in step (1) and the corresponding boronic acid are subjected to a Sizuki reaction or a substitution reaction with the corresponding aromatic amine to obtain the final product.
[0036] Method Two:
[0037] (1) Reaction of brominated aromatic compound and diboronic acid pinacol ester through Suziki reaction to obtain corresponding boric acid ester intermediate;
[0038] (2) The intermediate obtained in step (1) and the corresponding brominated aromatic hydrocarbon are subjected to Sizuki reaction ...
Embodiment 1
[0043] (1) Synthesis of intermediate [2-bromo-4'-fluorodiphenyl sulfone]
[0044]
[0045]Put 10mmol of 2-bromobenzenesulfonyl chloride and 25mmol of fluorobenzene into a three-necked flask, stir and dissolve under a nitrogen atmosphere, add 13mmol of ferric chloride, and stir at 40°C for 70min. 50 mL of 2M hydrochloric acid was added to terminate the reaction. It was extracted three times with appropriate amount of dichloromethane, and the obtained organic phase was extracted three times with pure water. The organic phase was dried by adding anhydrous sodium sulfate, filtered, and rotary evaporated under reduced pressure to obtain 2-bromo-4'-fluorodiphenyl sulfone. Yield 90%.
[0046] (2) Synthesis of intermediate [2-bromo-4'-phenothiazine diphenyl sulfone]
[0047]
[0048] Under the protection of a nitrogen atmosphere, 10 mmol of phenothiazine was added into a three-necked flask, and 20 mL of N,N-dimethylformamide was added and stirred to dissolve it. Add 10 mmol ...
Embodiment 2
[0053] (1) Synthesis of intermediate [2-fluoro-4'-fluorodiphenylsulfone]
[0054]
[0055] Put 10 mmol of 2-fluorobenzenesulfonyl chloride and 25 mmol of fluorobenzene into a three-necked flask, stir and dissolve under a nitrogen atmosphere, add 13 mmol of ferric chloride, and stir at 40°C for 70 minutes. 50 mL of 2M hydrochloric acid was added to terminate the reaction. It was extracted three times with appropriate amount of dichloromethane, and the obtained organic phase was extracted three times with pure water. The organic phase was dried by adding anhydrous sodium sulfate, filtered, and rotary evaporated under reduced pressure to obtain 2-fluoro-4'-fluorodiphenyl sulfone. Yield 90%.
[0056] (2) Synthesis of intermediate [2-fluoro-4'-phenothiazine diphenyl sulfone]
[0057]
[0058] Under the protection of a nitrogen atmosphere, 10 mmol of phenothiazine was added into a three-necked flask, and 20 mL of N,N-dimethylformamide was added and stirred to dissolve it. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com