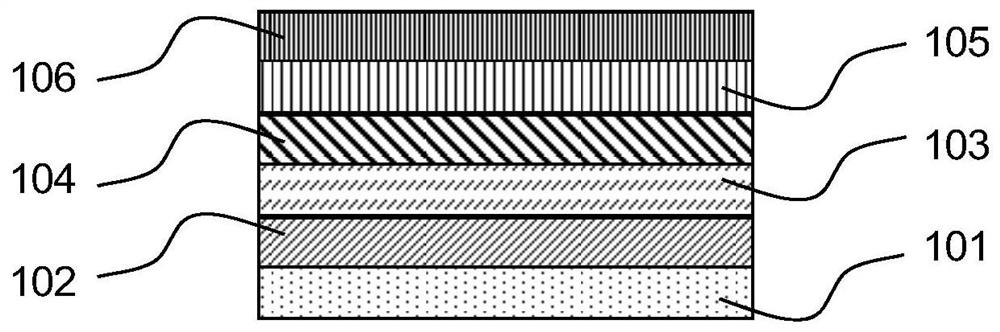
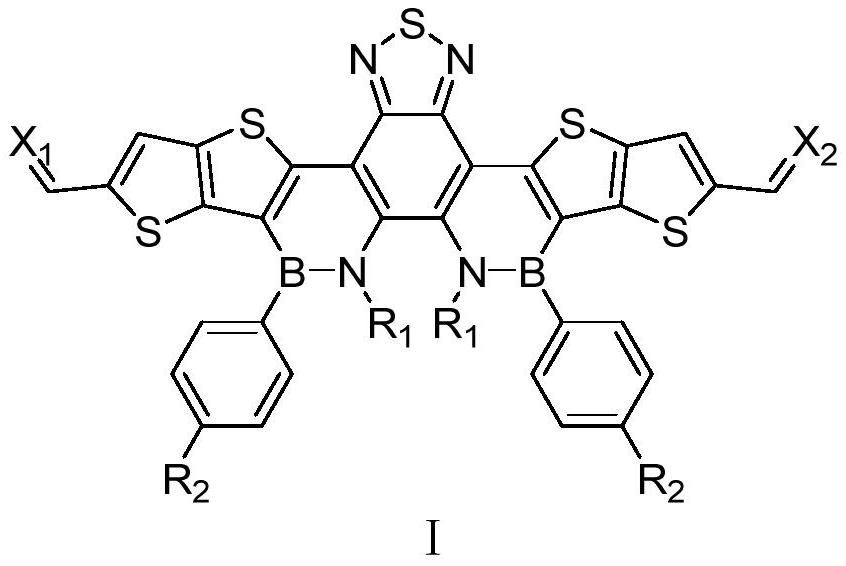
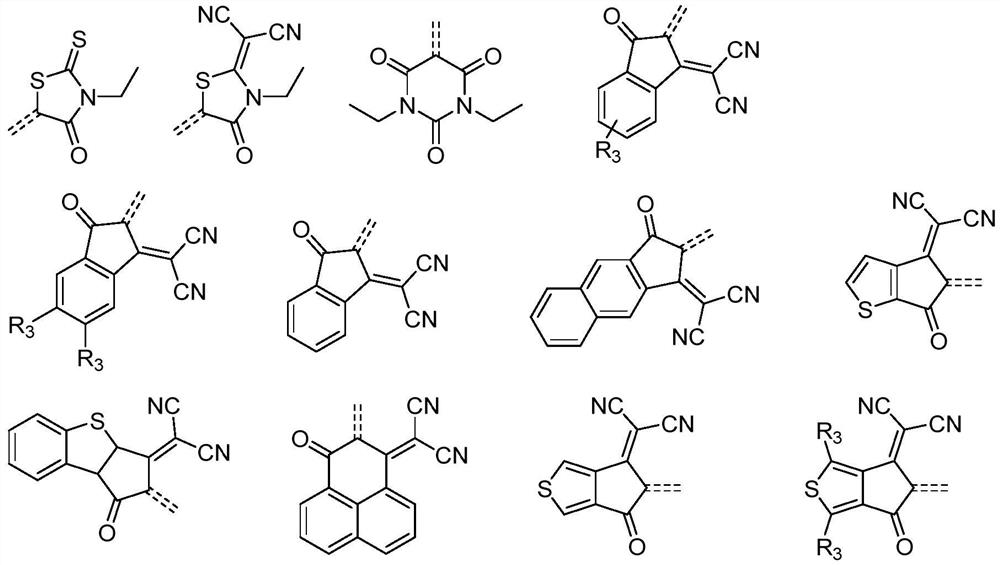
Benzothiadiazole boron-nitrogen derivative material and application thereof in organic electronic device
A technology of benzothiadiazole boron nitrogen and organic electronic devices. It is applied in the field of solar energy. It can solve the problems of affecting molecular film formation and accumulation, reducing device efficiency, and battery efficiency, achieving high photoelectric conversion efficiency, improving battery efficiency, Effect of Reducing Recombination Loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of compound 1
[0029]
[0030] Add 1-1 (6.04g, 6mmol), 1-2 (3.22g, 20mmol) and 5mL pyridine into a 500mL two-necked flask, then pump argon three times, add 250mL chloroform under the protection of argon, and react at 65°C 24 hours. Cool to room temperature, extract three times with dichloromethane, combine the organic phases, dry filter and add silica gel to spin dry, separate and purify by column chromatography (n-hexane:dichloromethane=3:1) to obtain 3.18g of white powder, yield 41% . MS(EI)m / z:[M]+:1292.35.
Embodiment 2
[0031] Embodiment 2: the synthesis of compound 2
[0032]
[0033] Add 1-1 (5.03g, 5mmol), 1-3 (3.68g, 16mmol) and 5mL pyridine into a 500mL two-neck flask, then pump argon three times, add 300mL chloroform under the protection of argon, and react at 65°C for 24 hours . Cool to room temperature, extract three times with dichloromethane, combine the organic phases, dry filter and add silica gel to spin dry, separate and purify by column chromatography (n-hexane:dichloromethane=3:1) to obtain 2.72 g of white powder, yield 38% . MS(EI)m / z:[M]+:1430.42.
Embodiment 3
[0034] Embodiment 3: the synthesis of compound 3
[0035]
[0036] Add 1-1 (6.03g, 6mmol), 1-4 (5.04g, 20mmol) and 5mL pyridine into a 500mL two-necked flask, then pump argon three times, add 300mL chloroform under the protection of argon, and react at 65°C for 24 hours . Cool to room temperature, extract three times with dichloromethane, combine the organic phases, dry filter and add silica gel to spin dry, separate and purify by column chromatography (n-hexane:dichloromethane=3:1) to obtain 3.27g of white powder, yield 37% . MS(EI) m / z:[M]+:1474.43.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com