Quadrivalent platinum anticancer complex containing ursolic acid ligand derived from cisplatin and its preparation method and application
A complex, tetravalent platinum technology, applied in the direction of steroids, anti-tumor drugs, drug combinations, etc., to achieve low toxicity, excellent anti-tumor effect, and the effect of overcoming cisplatin resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Preparation of Tetravalent Platinum Anticancer Complex Based on Cisplatin Derivation
[0053] All reagents and solvents were purchased from commercial suppliers. With tetramethylsilane as internal standard, record 1H NMR (300 or 500MHz) and 13 C NMR (125 MHz) spectrum. Analytical and preparative thin-layer chromatography was performed on silica gel (200-300 mesh) GF / UV 254 plates, and the chromatograms were visualized under UV light at 254 nm.
[0054] 1.1 Synthesis of intermediates
[0055] 1.1.1 Synthesis of platinum (IV) intermediates
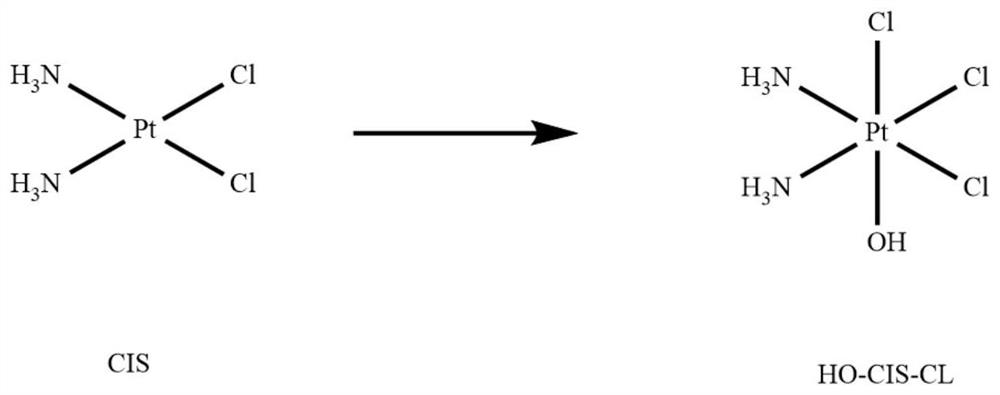
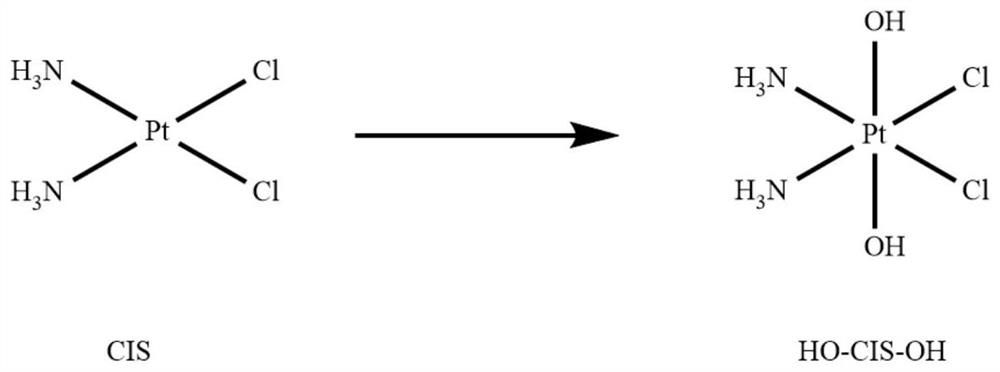
[0056] The preparation of the platinum (IV) intermediate takes the corresponding divalent cisplatin as the raw material and prepares it through the oxidation of N-chlorosuccinimide (NCS) or hydrogen peroxide.
[0057] The synthetic route of platinum (IV) intermediate monochloromonohydroxycisplatin see figure 1 , whose detailed synthesis method is as follows: To an aqueous solution (450 mL) of cisplatin (8.0mmoL, 1eq), N...
Embodiment 2
[0070] Identification of Example 2 Compounds
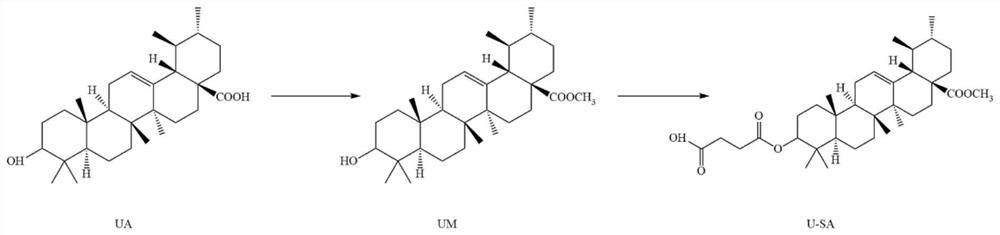
[0071] 2.1 Identification of compound UM
[0072] ESI-MS:m / z[M+H] + =471.5, the purity is 96%, proton nuclear magnetic spectrum (see Figure 7 ) and carbon spectra (see Figure 8 ). The details are as follows: 1 HNMR (300MHz, CDCl 3):δ=5.21(m,1H,CH(12)),3.58(s,3H,CH3(31)),3.18(dd,J=11.0,4.9Hz,1H,CH(3)),2.21(d ,J=11.3Hz,1H,CH(18)),1.98(ddd,J=13.4,13.4,4.6Hz,1H,Cha(2)),1.89(dd,J=8.9,3.7Hz,2H,CH2( 11)), 1.77 (ddd, J=13.8, 13.8, 4.8Hz, 1H, Cha(15)), 1.65–1.54 (m, 5H, CH2(7)+Cha(1)+CHa(16)+CHb( 2)),1.47(m,5H,CH(9)+Cha(6)+Cha(21)+Cha(22)+CHb(16)),1.36-1.25(m,4H,CH(19)+CHb (6)+CHb(22)+CHb(21)), 1.19–1.09(m,1H,CHb(15)),1.05(s,3H,CH3(27)),1.03–0.97(m,2H,CH (20)+CHb(1)),0.96(s,3H,CH3(23)),0.92(d,J=6.0Hz,3H,CH3(30)),0.90(s,3H,CH3(25)) ,0.83(d,J=6.4Hz,3H,CH3(29)),0.76(s,3H,CH3(24)),0.72(s,3H,CH3(26)),0.71(brd,J=10.4Hz ,1H,CH(5))ppm; 13 CNMR (125MHz, CDCl3): δ = 177.95 (C = 0, C28), 138.14 (C = CH, C13), 125.57 (HC = C, C12), 78.96...
Embodiment 3
[0082] Example 3 Stability and reducibility of tetravalent platinum anticancer complexes derived from cisplatin
[0083] 3.1 Preparation of solution
[0084] 3.1.1 Preparation of ascorbic acid solution
[0085] Accurately weigh a certain mass of ascorbic acid, add PBS to dissolve and prepare ascorbic acid solution with a concentration of 10mM.
[0086] 3.1.2 Preparation of PBS solution containing platinum complex
[0087] Preparation of tetravalent platinum complex PBS solution: Precisely weigh a certain mass of tetravalent platinum complex US-CIS-CL or US-CIS-SU or US-CIS-OH, first add acetonitrile to dissolve and prepare a 10μM solution, and then use Dilute it with PBS solution to obtain a solution with a final concentration of 1 μM, which is used for stability incubation experiments.
[0088] Cisplatin PBS solution preparation: Accurately weigh a certain mass of cisplatin, add PBS solution containing 10% acetonitrile, vortex until fully dissolved, and prepare a cisplatin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com