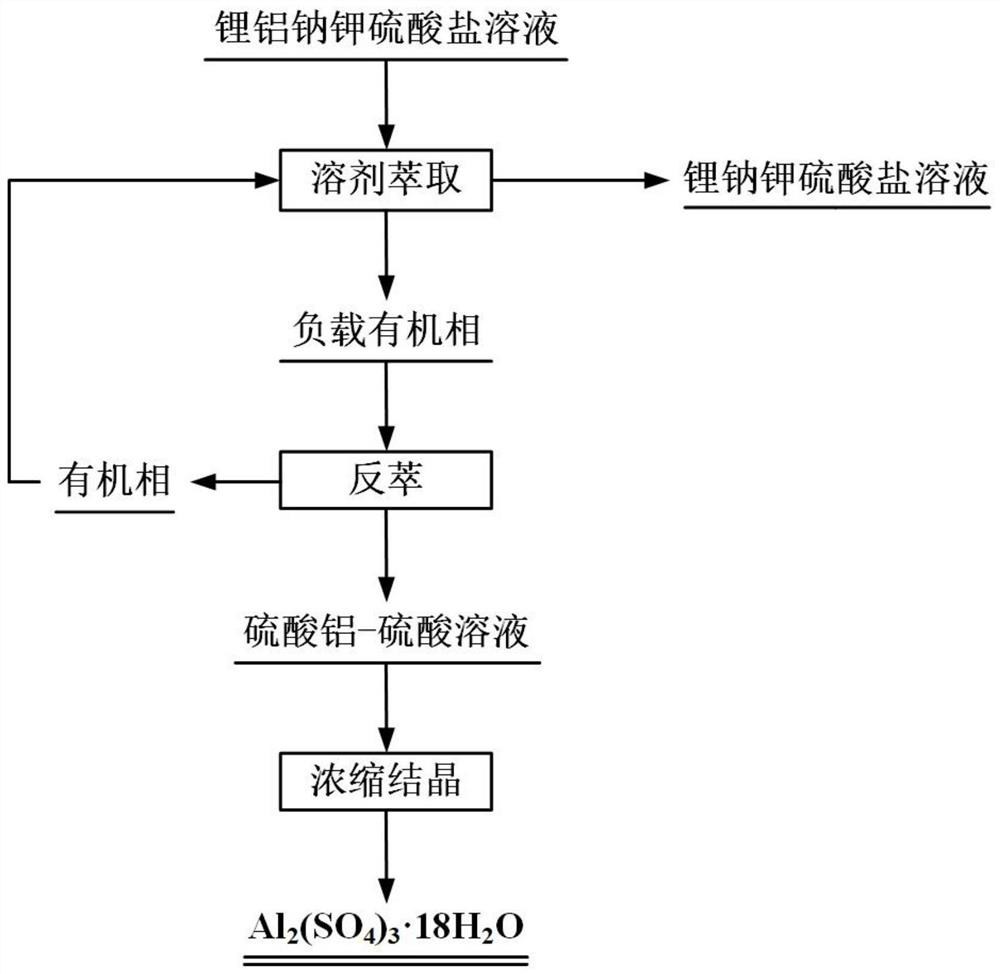
Method for selectively extracting and recovering aluminum from sulfate solution containing lithium, sodium, potassium and aluminum
A lithium sodium potassium aluminum and sulfate technology, applied in aluminum sulfate, lithium carbonate;/acid carbonate, alkali metal sulfite/sulfite, etc., can solve environmental pollution, ammonia nitrogen treatment difficulties, etc. problems, achieve high economic and environmental benefits, high stripping rate, and easy stripping and regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Mix bis(4-tert-octylphenyl) phosphate, n-butanol and sulfonated kerosene at a volume ratio of 40:10:50 to obtain an extracted organic phase. The lithium sodium potassium aluminum sulfate solution that preparation pH value is 4.5, wherein, the concentration of aluminum ion is 12.5g / L, and the concentration of lithium ion is 3.6g / L, and the concentration of sodium ion is 18g / L, and the concentration of potassium ion is 12g / L.
[0038] The extracted organic phase and lithium sodium potassium aluminum sulfate solution were mixed uniformly according to the volume ratio of 3:1, and subjected to three-stage countercurrent extraction at 40°C for 8 minutes, and then clarified and separated to obtain the loaded organic phase and lithium sodium potassium sulfate solution. saline solution. Use an equal volume of water at 40°C to carry out one-stage countercurrent washing on the loaded organic phase, clarify the layers, and mix the water washing solution and the raffinate.
[0039]...
Embodiment 2
[0043] Mix the extractant, n-butanol and sulfonated kerosene with a volume ratio of 40:10:50 to obtain the extracted organic phase. The extractant contains (4-tert-octylphenyl) phosphate and diphosphate with a mass ratio of 15:85 (4-tert-octylphenyl) ester. The lithium sodium potassium aluminum sulfate solution that preparation pH value is 2, wherein, the concentration of aluminum ion is 9.36g / L, and the concentration of lithium ion is 2.46g / L, and the concentration of sodium ion is 15g / L, and the concentration of potassium ion is 12.5g / L.
[0044] The extracted organic phase and lithium sodium potassium aluminum sulfate solution were mixed uniformly according to the volume ratio of 1:1, and subjected to 5-stage countercurrent extraction at 30°C for 10 minutes, and then clarified and separated to obtain the loaded organic phase and lithium sodium potassium sulfate solution. saline solution. The loaded organic phase with a volume ratio of 5:1 was mixed with water, and the loa...
Embodiment 3
[0048] Mix the extractant, isobutanol and sulfonated kerosene with a volume ratio of 30:5:65 to obtain the extracted organic phase. The extractant contains (dodecylphenyl) phosphate and di(dodecylphenyl) phosphate in a mass ratio of 10:90 dodecylphenyl) ester. Prepare a lithium sodium potassium aluminum sulfate solution with a pH value of 3, wherein the concentration of aluminum ions is 6.13g / L, the concentration of lithium ions is 1.95g / L, the concentration of sodium ions is 8.53g / L, and the concentration of potassium ions It is 6.21g / L.
[0049] The extracted organic phase and lithium sodium potassium aluminum sulfate solution were mixed uniformly according to the volume ratio of 1:1, and subjected to 4-stage countercurrent extraction at 25°C for 12 minutes, and then clarified and separated to obtain the loaded organic phase and lithium sodium potassium sulfate solution. saline solution. The loaded organic phase with a volume ratio of 2:1 was mixed with water, and the load...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com