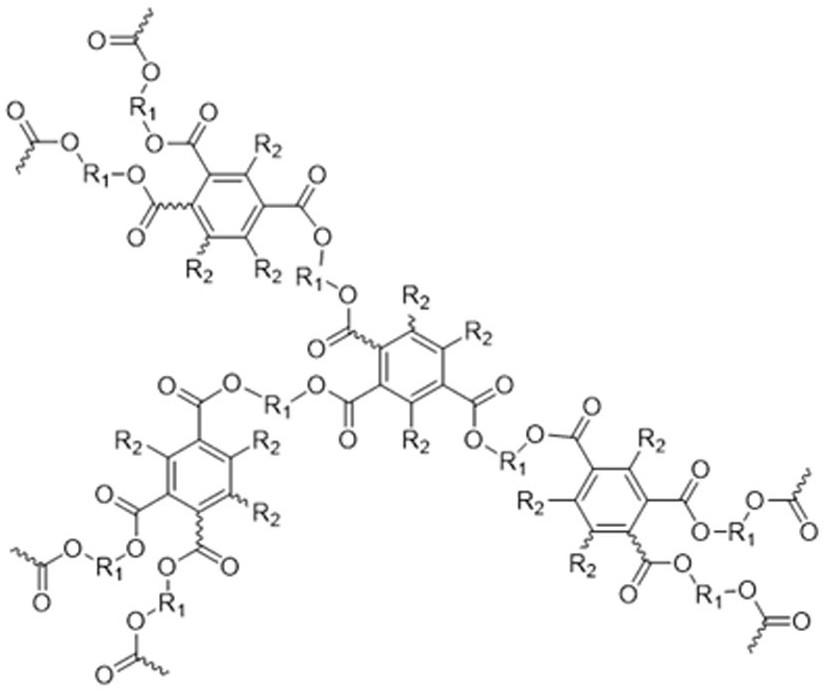
Hyperbranched polybenzoate as well as preparation method and application thereof
A technology of polybenzoate and endynoate, applied in the fields of polymer chemistry and material science, can solve the problems of less research on polymer materials, difficult monomer preparation and storage, etc., to prevent explosion attacks and achieve high thermal stability. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] (1) Synthesis of monomers
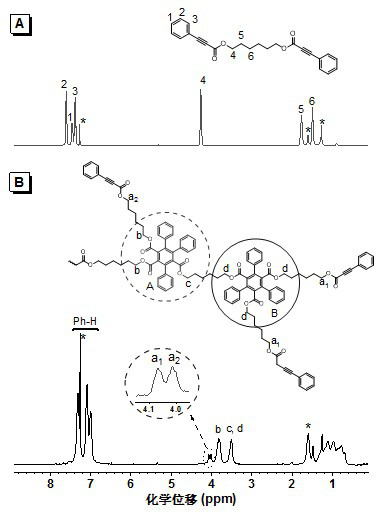
[0041] Add 1.18 g (10 mmol) of 1,6-hexanediol, 0.190 g (1.0 mmol) of p-toluenesulfonic acid, and 3.65 g (25 mmol) of phenylpropiolic acid into a 250 mL two-necked flask equipped with an oil-water separator, Add 100 mL of toluene, heat and reflux for 24 hours, concentrate the reaction solution, extract with dichloromethane, wash with aqueous sodium bicarbonate solution, dry the organic phase with anhydrous magnesium sulfate, distill the solvent under reduced pressure after filtration, and separate by silica gel column chromatography. It was a mixed solvent of petroleum ether / ethyl acetate (20:1, v / v), and the product was vacuum-dried to constant weight to obtain 2.40 g of a white solid (yield 64.2%), which was a phenylpropiolic acid dibasic ester monomer. 1 H NMR (400 MHz, CDCl 3), δ (TMS, ppm): 7.59 (d, 4H), 7.45 (t, 2H), 7.37 (t, 4H),4.24 (t, 4H), 1.75 (q, 4H), 1.47 (p, 4H) ;
[0042] (2) Preparation of polymer
[0043] Add 3...
Embodiment 2
[0045] Phenylpropiolic acid dibasic ester monomer is with embodiment 1. Add 37.4 mg (0.10 mmol) of phenylpropiolic acid dibasic ester monomer, 3.2 mg (0.012 mmol) of rhodium chloride hydrate to a 10 mL polymerization tube with a sidearm. Pump N into the polymer tube through the side arm 2 Three times, add 0.6 mL redistilled toluene and 3.9 mg (0.03 mmol) of diisopropylethylamine, stir to dissolve. The system was reacted at 115 °C for 15 h. After the reaction was completed, it was cooled to room temperature, diluted with 3 mL of chloroform, and added dropwise to 150 mL of conventionally stirred methanol through a dropper plugged with cotton. Stand still, filter, and dry at room temperature to constant weight to obtain a polymer. Yield: 50.8%. Gel permeation chromatography (GPC) results showed that the weight average molecular weight ( M w ) was 20500, and the molecular weight distribution (PDI) was 2.15.
Embodiment 3
[0047] Phenylpropiolic acid dibasic ester monomer is with embodiment 1. Add 37.4 mg (0.10 mmol) of phenylpropiolic acid dibasic ester monomer, 3.2 mg (0.012 mmol) of rhodium chloride hydrate to a 10 mL polymerization tube with a sidearm. Pump N into the polymer tube through the side arm 2 Three times, add 0.6 mL redistilled toluene and 3.9 mg (0.03 mmol) of diisopropylethylamine, stir to dissolve. The system was reacted at 115 °C for 20 h. After the reaction, it was cooled to room temperature, diluted with 3 mL of chloroform, and added dropwise to 150 mL of conventionally stirred methanol through a dropper plugged with cotton. Stand still, filter, and dry at room temperature to constant weight to obtain a polymer. Yield: 53.5%. Gel permeation chromatography (GPC) results showed that the weight average molecular weight ( M w ) was 23300, and the molecular weight distribution (PDI) was 2.11.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com