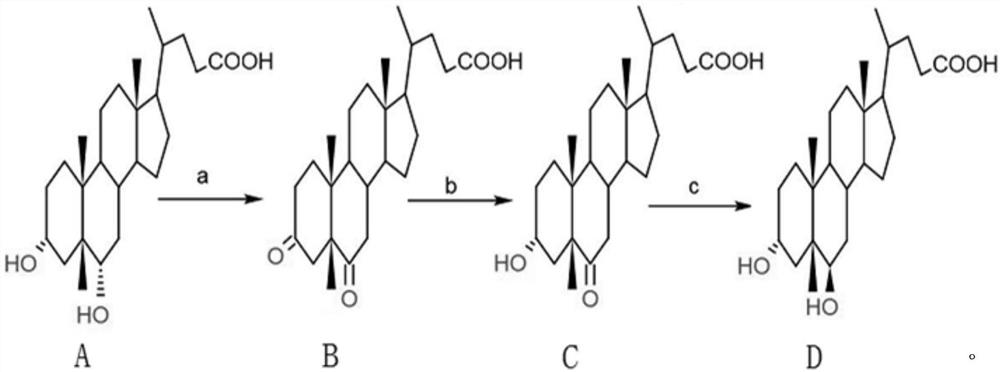
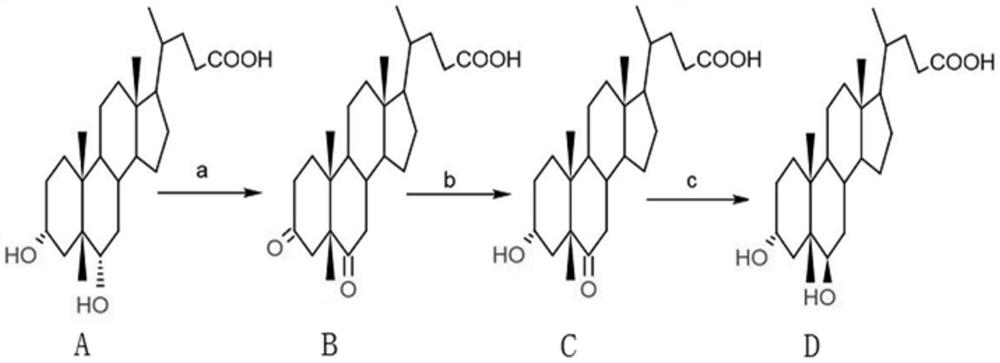
Preparation method of murine deoxycholic acid
A technology of deoxycholic acid and hyodeoxycholic acid, which is applied in the field of preparation of murine deoxycholic acid, and achieves the effects of high conversion rate, less side reactions and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation method of the murine deoxycholic acid of the present embodiment, comprises the steps:
[0034] a. Preparation of 3,6-dicarbonyl-5β-cholanic acid
[0035] Add 10g of hyodeoxycholic acid and 50ml of acetone into a 500ml reaction bottle, lower the temperature to below 0°C, slowly add 46ml of hypochlorous acid with a content of 10%, and continue to react for 30min after the addition is completed. The residual hyodeoxycholic acid detected by HPLC is 0.237 %, add 0.3g sodium bisulfite to terminate the reaction; distill off acetone, add 200ml water and stir for 30min to crystallize completely, filter, wash the filter cake with 2*250ml water, until the washing water is neutral by pH test paper, and 3,6- The wet product of dicarbonyl-5β-cholanic acid is about 12.5 g, and the purity detected by HPLC is 98.352%.
[0036] b. Preparation of 3α-hydroxy, 6-carbonyl-5β-cholanic acid
[0037] Dissolve the 3,6-dicarbonyl-5β-cholanic acid in the previous step in 100ml of...
Embodiment 2
[0041] a. Preparation of 3,6-dicarbonyl-5β-cholanic acid
[0042] Add 10g of hyodeoxycholic acid and 80ml of acetone into a 500ml reaction bottle, cool down to below 0°C, slowly add 13.4ml of Jones reagent (mixed solution of chromium trioxide and sulfuric acid), continue to react for 30min after adding, sample HPLC for detection of hyodeoxychol The acid residue was 0.127%, and 0.3 g of sodium bisulfite was added to terminate the reaction. Distill to remove acetone, add 200ml of water and stir for 30min to crystallize completely, filter, wash the filter cake with 2*250ml of water until the washed water is neutral by pH test paper, and the wet product of 3,6-dicarbonyl-5β-cholanic acid is about 12.7 g, the purity detected by HPLC is 98.257%.
[0043] b. Preparation of 3α-hydroxy, 6-carbonyl-5β-cholanic acid
[0044] Dissolve the 3,6-dicarbonyl-5β-cholanic acid in the previous step in 105ml of 1% sodium hydroxide solution, adjust the pH of the solution to 7.0-7.5, add 5.5g of g...
Embodiment 3
[0048] a. Preparation of 3,6-dicarbonyl-5β-cholanic acid
[0049] Add 10g of hyodeoxycholic acid and 100ml of acetone into a 500ml reaction bottle, lower the temperature to below 0°C, slowly add 40ml of saturated potassium permanganate solution, and continue the reaction for 30min after the addition is complete. The residual hyodeoxycholic acid is detected by sampling HPLC as 0.226%. 0.3 g of sodium bisulfite was added to terminate the reaction. Distill to remove acetone, add 200ml of water and stir for 30min to completely crystallize, filter, wash the filter cake with 2*250ml of water until the washing water is neutral by pH test paper, and the wet product of 3,6-dicarbonyl-5β-cholanic acid is about 13.2 g, the purity detected by HPLC is 98.066%.
[0050] b. Preparation of 3α-hydroxy, 6-carbonyl-5β-cholanic acid
[0051] Dissolve the 3,6-dicarbonyl-5β-cholanic acid in the previous step in 110ml of 1% sodium hydroxide solution, adjust the pH of the solution to 7.0-7.5, add 6g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com