Composite marker for diagnosis of age related macular degeneration and use thereof
A biomarker, macular degeneration technology, applied in the field of composite biomarkers, can solve problems such as reducing central vision, damage to photoreceptor cells, bleeding, etc., and achieve the effect of high diagnostic ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1: Choosing age-related macular degenerative patients and collecting plasma
[0102] The approval of the clinical trial review committee of the Pot Tangjrew University Hospital was obtained to collect the plasma samples of age-relevance macular transition patients. For the quantitative detection of biomarkers, a total of 184 plasma samples were analyzed by plasma proteomics. Table 1 shows the normal group (NON AMD) and age-related macular degenerative (AMD) disease groups as analytical objects. Clinical features.
[0103] Table 1
[0104] Sample clinical information
[0105]
Embodiment 2
[0106] Example 2: Select peptide and use multi-reaction monitoring mass spectrometry quantitative analysis peptide
[0107] 2-1: Select peptide and design synthetic peptide
[0108] In the present invention, as a biomarker for diagnosing age-related macular degeneration, 13 of the following: IGFBP2 (insulin-like growth factor binding protein 2), Ada MTSL2 (ADAMTS sample protein 2), CFH (supplementary factor) H), CP (Copper Blue Protein), DDI2 (Protein DDI1 Source 2), Fcn2 (Fibrin Glue 2), LGALS3B P (galactin 3 binding protein), MBL2 (mannose binding protein C), pnlip (Pancreatal trioin fat enzyme), SELE (E-selectin), Siglec14 (sialic acid binding immunoglobulin-like concentrate 14), THBS1 (platelet-free protein-1) and ZG16B (enzyme original particulate protein 16 homolog) B).
[0109] In order to perform multi-reaction monitoring analysis of the above biomarkers, representative peptides (Q1) having a talloid ratio (M / Z) for the protein specificity of 13 biomarkers were selecte...
Embodiment 3
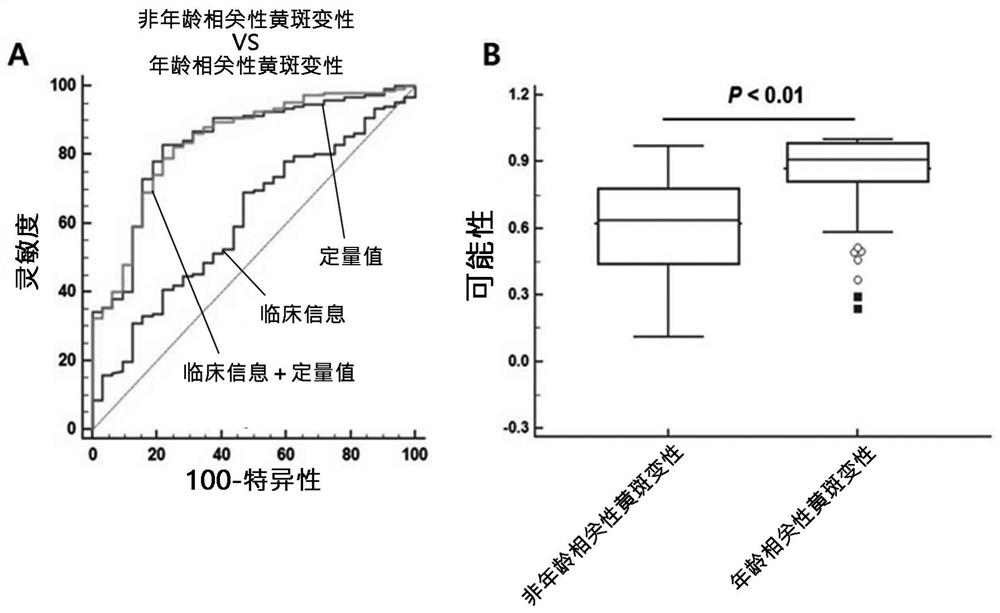
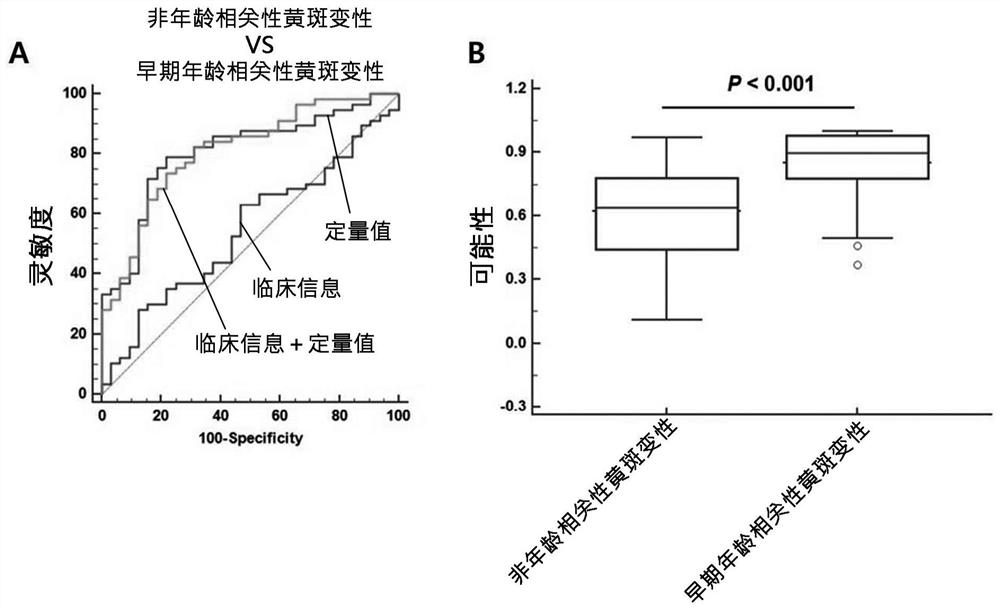
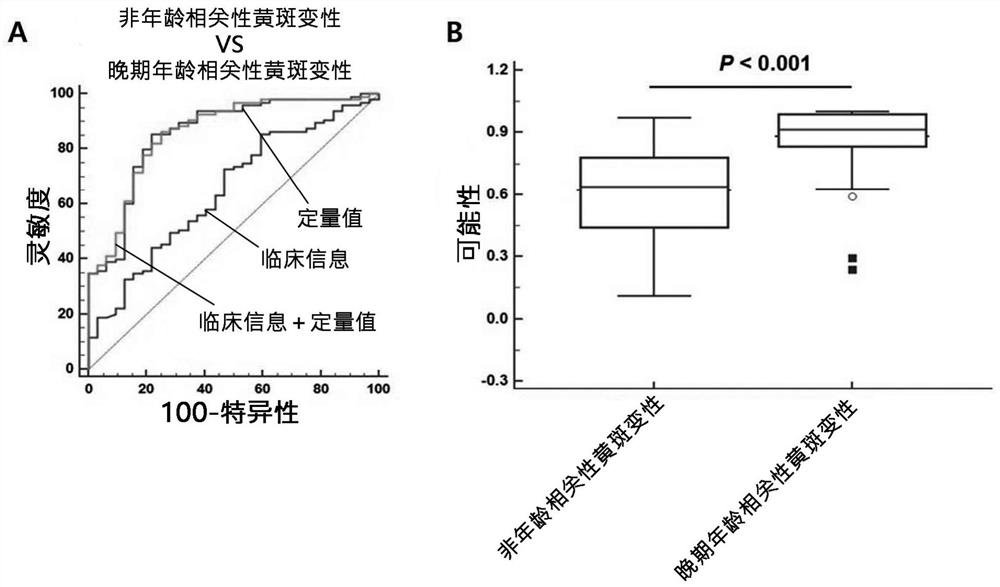
[0122] Example 3: Confirmation of the improvement of the diagnostic ability of the composite biomarker group and clinical information
[0123] In the present invention, when combined with 13 composite biomarker groups and clinical information, in order to confirm the improvement of the diagnostic ability, the degree of input biomarker expression quantity conversion information and clinical information conversion is used, thereby speculating classification The probability of age-related macular degeneration.
[0124] table 3
[0125] Confirm that the age-related macular degenerative diagnosis performance according to the composite biomarker binding
[0126]
[0127] 1. Clinical information, 2.IGFBP2, 3.adamtsl2,4.lgals3bp, 5.cfh, 6.thbs1 ,7.siglec14, 8.cp, 9.SELE, 10.DDI2, 11.M BL2, 12.PNLIP, 13.ZG16b, 14.fcn2
[0128] Table 4
[0129] Confirmation according to the diagnostic ability of the composite biomarker group and clinical information
[0130]
[0131] C: Clinical informat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com