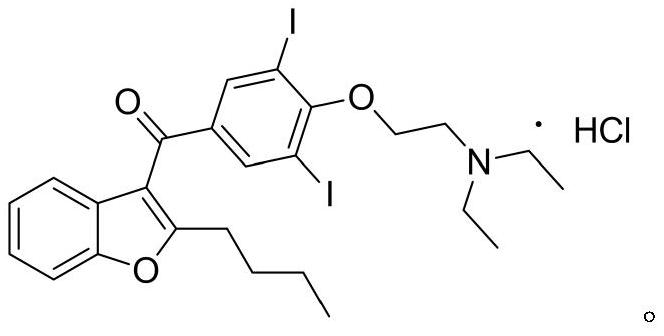
Method for preparing amiodarone hydrochloride
An amiodarone hydrochloride and acid-catalyzed technology, which is applied in the field of medicine, can solve the problems of difficult crystallization, high treatment costs, and low purification yield, and achieve the effects of reducing three wastes, fewer by-products, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
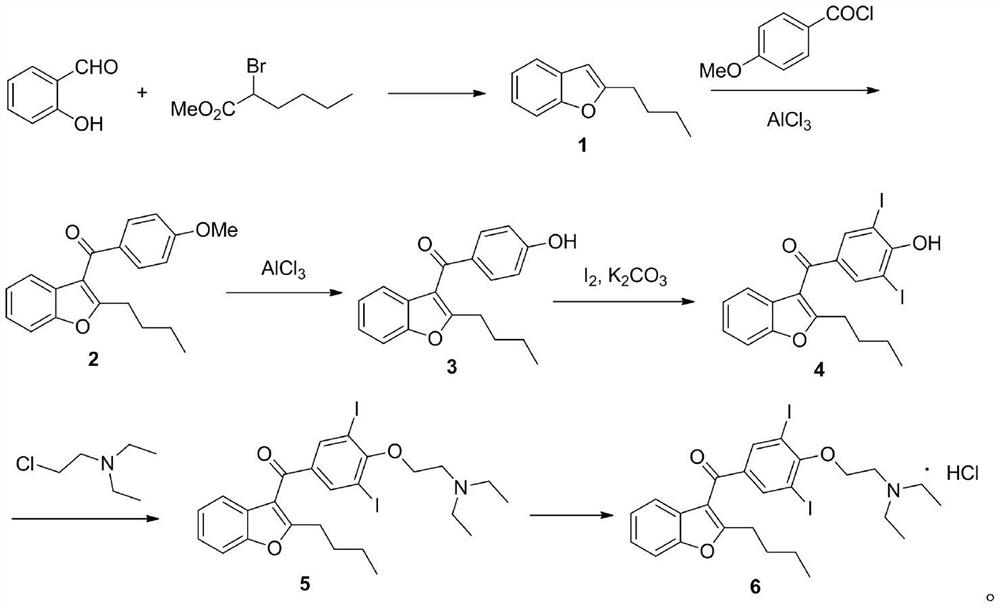
[0033] The preparation method of the present invention can be expressed as follows:
[0034]
Embodiment 1
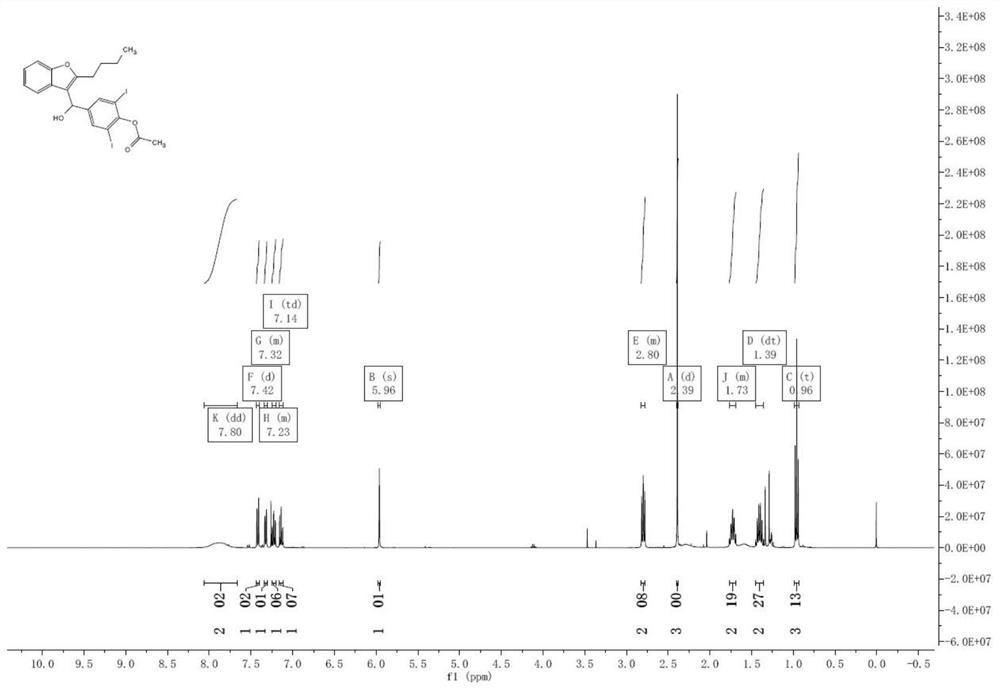
[0036] Dissolve 2-butylbenzofuran 1 (17.4g, 100mmol) in 1,2-dichloroethane (120mL), add anhydrous ferric chloride (1.62g, 10mmol), then add p-acetoxy Benzaldehyde (18.1g, 110mmol) was reacted at 80°C for 5 hours. After the reaction, 50 mL of water was added, stirred for 30 minutes, the organic layer was separated, washed with 20 mL of saturated sodium bicarbonate solution, and the solution was evaporated to dryness to obtain a crude compound of formula 8, which was directly used in the next reaction. 1 H NMR (400MHz, CDCl 3 )δ7.80(s,2H),7.42(d,J=8.0Hz,1H),7.34–7.31(m,1H),7.25–7.20(m,1H),7.14(td,J=7.6,1.0Hz ,1H),5.96(s,1H),2.79(t,J=7.6Hz,2H),2.39(s,3H),1.77–1.69(m,2H),1.45-1.36(m,2H),0.96( t,J=7.2Hz,3H).
[0037] The above crude compound of formula 8 was dissolved in methanol (200 mL), added elemental iodine (81.3 g, 0.32 mol) and sodium hydroxide (24.0 g, 0.60 mol), and reacted at 65° C. for 2 hours. After the reaction, add saturated sodium sulfite (10mL) and dilute hydroc...
Embodiment 2
[0040] Dissolve 2-butylbenzofuran 1 (17.4g, 100mmol) in dichloromethane (120mL), add anhydrous zinc chloride (10mmol), then add p-acetoxybenzaldehyde (18.1g, 110mmol), React at 90°C for 3 hours. After the reaction, 50 mL of water was added, stirred for 30 minutes, the organic layer was separated, washed with 20 mL of saturated sodium bicarbonate solution, and the solution was evaporated to dryness to obtain a crude compound of formula 8, which was directly used in the next reaction.
[0041] The above crude compound of formula 8 was dissolved in isopropanol (200 mL), added elemental iodine (81.3 g, 0.32 mol) and potassium hydroxide (0.60 mol), and reacted at 50° C. for 5 hours. After the reaction, add saturated sodium sulfite (10mL) and dilute hydrochloric acid (2M, 10mL) to wash, extract with dichloromethane, combine the organic layers and evaporate to dryness, then recrystallize with dichloromethane to obtain the compound of formula 4, the two-step yield is 67% .
[0042]T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com