Agkistrodon halys venom phospholipase A2 marker polypeptide and application thereof
A technology of snake venom phospholipids and pit vipers, applied in the field of chemical analysis and quantitative detection, can solve the problems of poor accuracy, cumbersome determination methods, inability to accurately characterize the quality of snake venom and its extracts, and achieve high detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] This example aims to screen characteristic peptides, using purified phospholipase A2 as an analyte, using a nanoliter liquid phase-high resolution mass spectrometer, the method focuses on obtaining abundant enzymatic peptides to ensure that the screening range has as high a coverage as possible Rate.
[0039] The preparation of the solution for feature polypeptide screening comprises the following steps:
[0040] Put 5 mg of Agkistrodon venom phospholipase A2 in a volumetric flask with a volume of 10 mL, then dissolve it with ammonium bicarbonate solution with a molar concentration of 25 mmol / L and dilute to 10 mL, and accurately measure 200 μL, Solution a is obtained.
[0041]Add 10 μL of dithiothreitol solution with a concentration of 0.2 mol / L to the solution a, mix well, react for 1 hour under the condition of heating in a constant temperature water bath at 60 °C, and then add ethyl iodide with a concentration of 0.2 mol / L Amide solution 20 μL, protected from ligh...
Embodiment 2
[0046] The "test solution" described in this example is based on the detection of the target characteristic polypeptide WDDYTYSWK screened in Example 1, which maximizes the enzymatic hydrolysis efficiency of the sample and does not need to consider other enzymatic hydrolysis peptides, so the optimized method There is no need to use dithiothreitol and iodoacetamide in Example 1 for treatment.
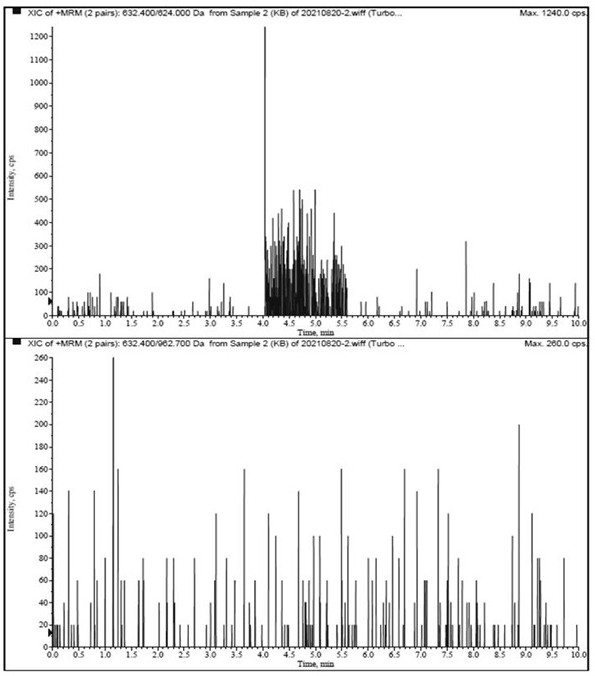
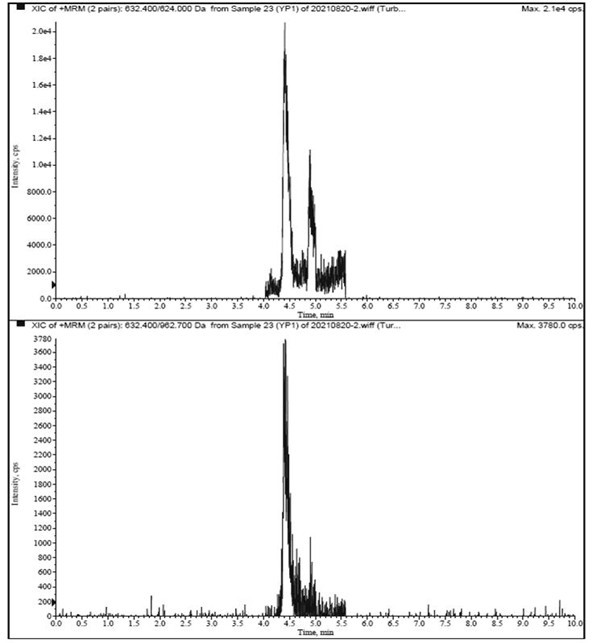
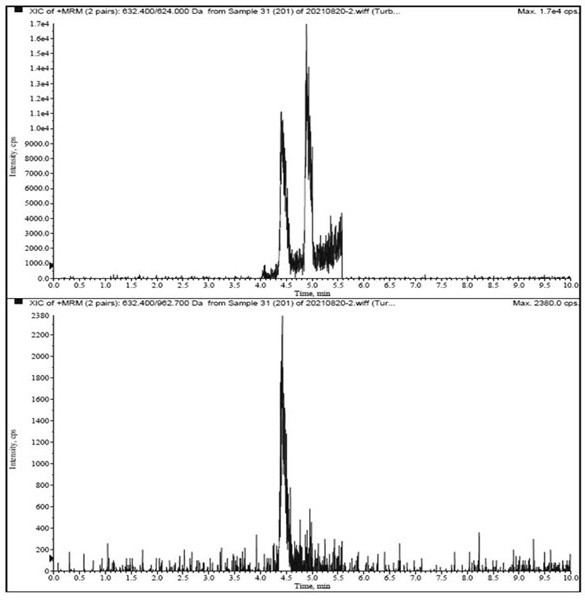
[0047] Multiple reaction monitoring (MRM) quantitative analysis of Agkistrodon venom phospholipase A2 in samples.
[0048] 1. Instruments and equipment: SCIEX Triple Quad 6500 triple quadrupole mass spectrometer, CP225D electronic balance (Sartorius, Germany), Sigma3-30K refrigerated centrifuge (Sigma, Germany), Milli-QAdvantageA10 ultrapure water instrument (Millipore, USA ).
[0049] 2. Chromatography and mass spectrometry conditions:
[0050] (1) Liquid conditions: Agilent Poroshell 120, SB-C18 column (100 mm×2.1 mm, 1.7 μm); column temperature: 40 ℃; injection volume: 10 μL; flow r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com