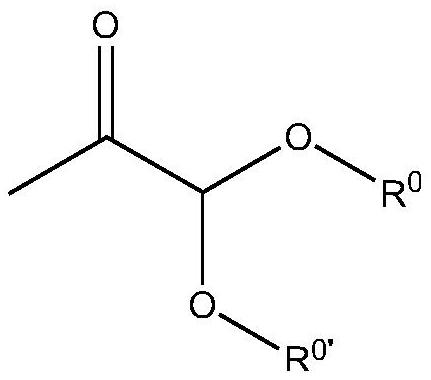
Preparation method of pyruvic aldehyde glycol
A technology for acetal acetal and dihydroxyacetone is applied in the field of preparing acetal acetal, and can solve the problems of harsh reaction conditions, difficult separation of by-products, expensive raw materials and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] The preparation method of the catalyst of the present invention may comprise the following steps: the sodium sulfonate type cation exchange resin cleaned by deionized water and ethanol is stirred and soaked (1~24h) with sulfuric acid or hydrochloric acid (0.5~20% concentration for example), deionized water Wash with ethanol to neutrality to obtain the sulfonic acid type cation exchange resin of the present invention.
[0074] In some embodiments, the mass ratio of the 1,3-dihydroxyacetone to the alcohol is 1:0.01-1:100 (for example, 1:0.01, 1:0.02, 1:0.03, 1:0.04, 1:0.01 0.05, 1:0.1, 1:0.5, 1:1, 1:2, 1:3, 1:4, 1:5, 1:6, 1:7, 1:8, 1:9, 1:10, 1:11, 1:12, 1:13, 1:14, 1:15, 1:16, 1:17, 1:18, 1:19, 1:20, 1:25, 1:30, 1:25 35, 1:40, 1:45, 1:50, 1:55, 1:60, 1:65, 1:70, 1:75, 1:80, 1:85, 1:90, 1:95, 1:100, etc.), or 1:0.1-10:1.
[0075] The molar concentrations of the raw materials 1,3-dihydroxyacetone and alcohol are respectively 0.01 mol / liter to 20 mol / liter. The form of ...
Embodiment 1
[0087] Synthesis of Dimethyl Acetal of Aceguvaldehyde
[0088] Add 1.5 grams of catalyst hydrogen sulfonic acid cation exchange resin, 13.6 grams of 1,3-dihydroxyacetone and 150 milliliters of methanol in a 300 milliliter tank reactor (the molar concentration of 1,3-dihydroxyacetone and methanol is 1.0 mol / liter ), reacted at 110°C and 0.1-0.7MPa pressure for 10 hours, and took samples for analysis by gas chromatography. The molar yield of the prepared aceguvaldehyde dimethyl acetal is 55% (based on 1,3-dihydroxyacetone), and the selectivity is 65%.
Embodiment 2
[0090] Add 2.0 g of Sn ion-modified sulfonic acid type cation exchange resin catalyst, 13.6 g of 1,3-dihydroxyacetone and 280 ml of isooctyl alcohol into a 300 ml tank reactor, and react at 35 °C and 0.02-0.3 MPa After 36 hours, a sample was taken and analyzed by gas chromatography. The molar yield of diisooctanol acetal acetal obtained is 83% (based on 1,3-dihydroxyacetone), and the selectivity is 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com