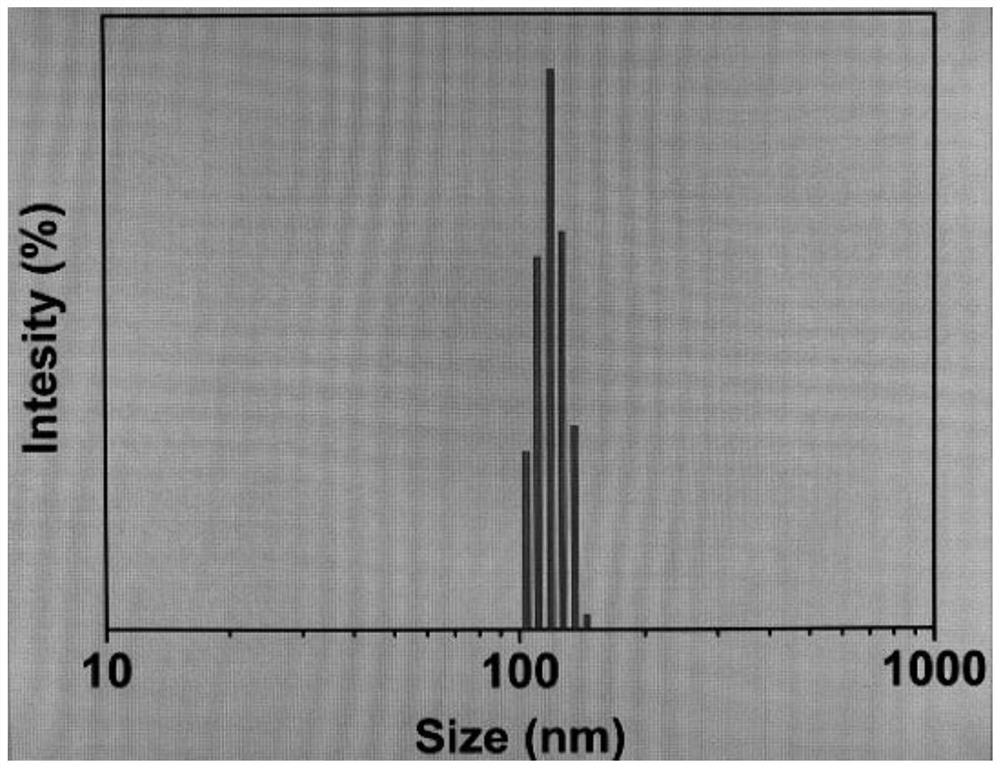
Method for preparing amphiphilic block copolymer through cooperation of light-operated free radical polymerization and ring-opening copolymerization
A technology of block copolymers and free radicals, applied in the field of synthesis of amphiphilic polymers, can solve problems such as the inability to realize the regulation of the molecular weight of the two blocks and limited types of monomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
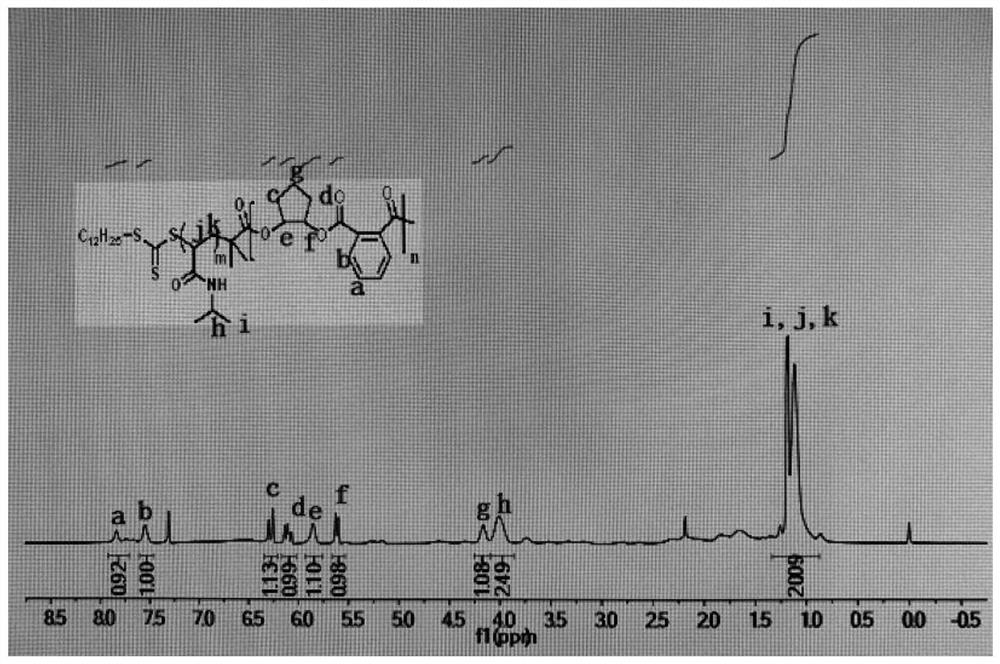
[0034] In a nitrogen-filled glove box, a stirrer was placed in the dry polymerization reaction tube in turn, and the chain transfer agent initiator 4-cyano-4-[(dodecylsulfanylthiocarbonyl)thiocarbonyl]valeric acid was added. (TTC-COOH) (80mg, 0.2mmol), (2,4,6-trimethylbenzoyl)diphenylphosphine oxide (TPO) (17.4mg, 0.05mmol), N-isopropylacrylamide ( 1.13g, 10mmol), cyclopentane oxide (0.44mL, 5mmol), phthalic anhydride (recrystallization and then sublimation) (0.74g, 5mmol), THF solution of triethylboron (TEB) (10μL, 0.1 mmol), phosphazene base (t-BuP1) (10 μL, 0.04 mmol), THF (2 ml). After the feeding is completed, shake evenly to completely dissolve the monomer, chain transfer agent, initiator and catalyst. Take it out and put it in a 60°C oil bath to react under magnetic stirring, while using 3mw / cm 2 The intensity was 405nm wavelength blue light irradiation while heating for 24 hours. After the reaction, a small amount of crude product was taken for nuclear magnetic test ...
Embodiment 2
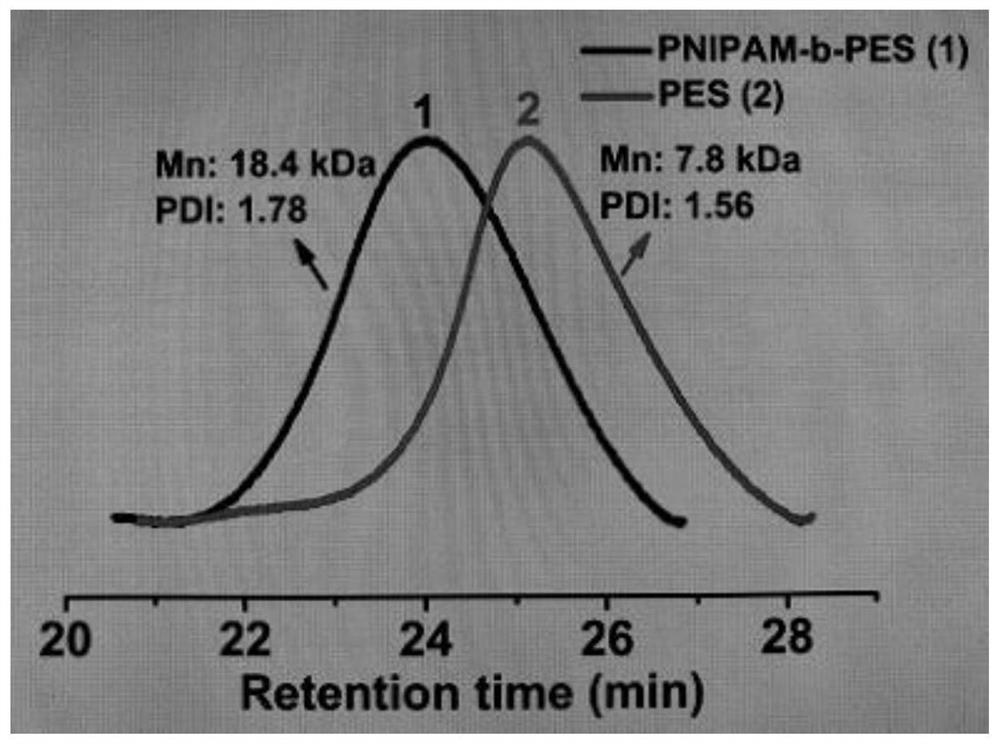
[0037] Other polymerization conditions in this example are the same as in Example 1, and 4-cyano-4-[(dodecylsulfanylthiocarbonyl)thiocarbonyl]valeric acid (TTC-COOH) is still used as chain transfer agent initiator , the different blue light irradiation time was changed to 0h, the heating time was unchanged, the reaction was carried out in an oil bath at 60°C for 24h, the categories and proportions of other materials were the same, the conversion rate of phthalic anhydride monomer was >99%, and the molecular weight of the obtained polymer was 7.8kDa, molecular weight distribution is 1.56, only heating without light, see attached figure 1 Medium data 2.
Embodiment 3
[0039] Other polymerization conditions in this example are the same as in Example 1, and 4-cyano-4-[(dodecylsulfanylthiocarbonyl)thiocarbonyl]valeric acid (TTC-COOH) is still used as chain transfer agent to initiate The difference is that the blue light intensity is unchanged and the irradiation time is changed to 12h, the heating time is unchanged, and the reaction is carried out in an oil bath at 60°C for 24h. The conversion rate of phthalic anhydride monomer is >99%, the molecular weight of the obtained polymer is 14.7kDa, and the molecular weight distribution is 1.64.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com