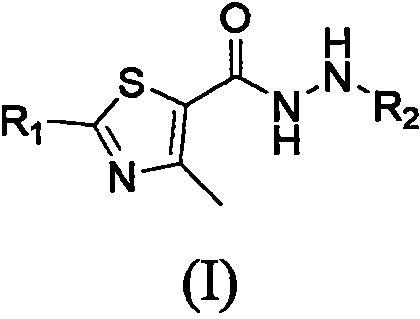
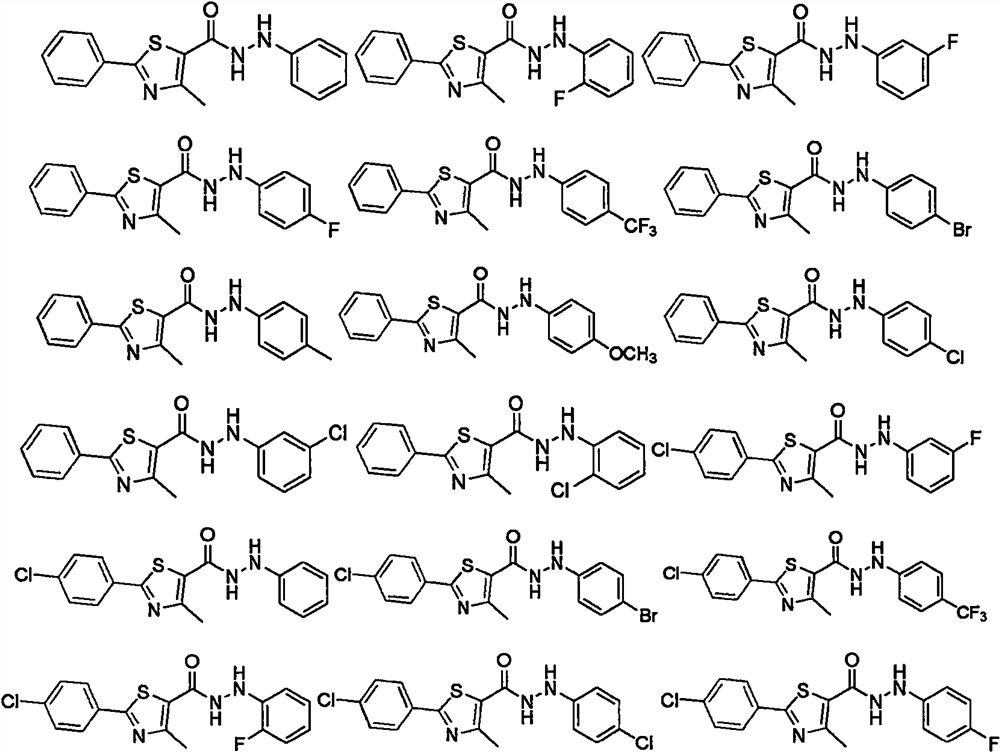
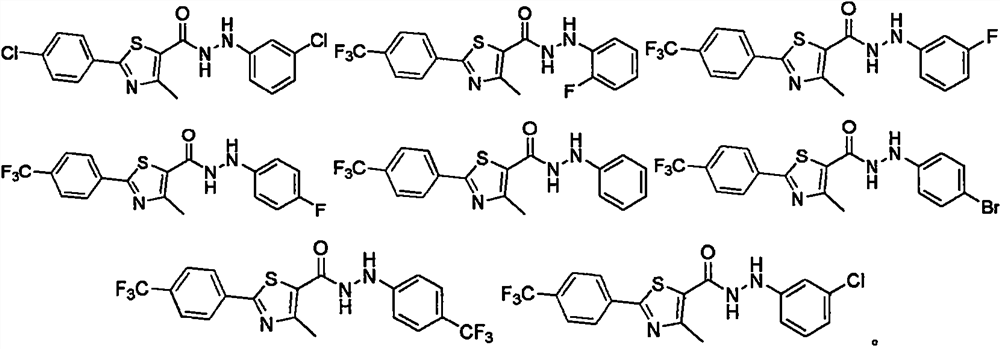
Thiazole hydrazide compound as well as preparation method and application thereof
A technology for thiazole hydrazides and compounds, which is applied in the field of thiazole hydrazides and their preparations, and can solve the problems of crop yield decline, farmers' economic losses, and quality reduction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: Preparation of ethyl 4-methyl-2-phenylthiazole-5-carboxylate
[0049] Take 5g (36.44mmol) of thiobenzamide and add it to a 150mL round-bottomed flask, then add 6.05mL (43.73mmol) of ethyl 2-chloroacetoacetate and 60mL of ethanol and raise the temperature to 100°C for about 6 hours under reflux. TLC monitors the reaction process. After the reaction is completed, the reaction is stopped; ethanol is removed by distillation under reduced pressure, and the 200mL EA dissolving system is washed 3 times with 100mL water. / EA=30 / 1) to obtain the product, 5.69 g of yellow liquid, the yield was 63.2%.
[0050] For other ester intermediate compounds, corresponding raw materials or substituents are used, and are synthesized with reference to the steps of Example 1.
Embodiment 2
[0051] Embodiment 2: Preparation of 4-methyl-2-phenylthiazole-5-carboxylic acid
[0052] Take 1g (36.44mmol) of ethyl 4-methyl-2-phenylthiazole-5-carboxylate and put it into a 100mL round bottom flask, add 2 times the amount of sodium hydroxide solution, and use 20mL of methanol as a solvent. After reacting for about 6 hours, TLC monitored the reaction process. After the reaction was completed, the reaction was stopped; methanol was distilled and desolvated under reduced pressure, concentrated hydrochloric acid was added to the system, the pH was adjusted to 2-3, suction filtered, and washed 3 times with ice water (5mL* 3), the filter cake was dried under an infrared lamp to obtain 0.61 g of a white solid with a yield of 68.8%.
[0053] Other carboxylic acid intermediate compounds are synthesized with reference to the steps of Example 2 using corresponding raw materials or substituents.
Embodiment 3
[0054] Example 3: Preparation of 4-methyl-2-phenylthiazole-N'-phenyl-5-formylhydrazide
[0055] Take 0.2g (0.91mmol) 4-methyl-2-phenylthiazole-5-carboxylic acid and add it to a 15mL pressure bottle, then add 0.35g (1.83mmol) EDCI, 0.15g (1.09mmol) HOBT, 3mL DCM Dissolve the system, add 0.14g (0.92mmol) phenylhydrazine at last, stir and react at room temperature, react for about 5h, monitor the reaction process by TLC, stop the reaction after the reaction is completed; dissolve the compound in 150mL DCM system, wash 3 times with 50mL water, add the organic phase Dry over anhydrous sodium sulfate, filter with suction, distill under reduced pressure to obtain a light yellow solid, add absolute ethanol for recrystallization, and precipitate a light yellow solid, filter with suction, wash with a small amount of absolute ethanol, and dry the compound under an infrared lamp After drying, it was weighed to obtain 0.08 g, and the yield was 28.4%. The melting point is 166.5-167.8°C.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Ec50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com