Csf1r inhibitors for use in treating cancer
A dose, patient technology, applied in the direction of medical preparations containing active ingredients, drug combinations, organic active ingredients, etc., can solve problems such as high recurrence rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Embodiment 1. The synthetic route of compound 1
[0155] Compound A: 3-((2-chloropyridin-4-yl)oxy)-6-iodo-2-methylpyridine
[0156]
[0157] A solution of 3-hydroxy-2-picoline (20.0 g, 183) and Na2CO3 (38.8 g, 367 mmol) in H2O (320 mL) and MeOH (200 mL) was treated with I2 (46.5 g, 183 mmol) and incubated at room temperature Stir for 1 hour. The mixture was acidified with HCl (2M), extracted with EtOAc (2x), and the combined organics were washed with brine, dried over Na2SO4 and concentrated to dryness. This material was suspended in 1:1 EtOAc / Hex, sonicated and the solid collected via filtration and dried. The filtrate was concentrated to dryness, treated with DCM, the solid was collected via filtration and combined with the first solid to give 6-iodo-2-methylpyridin-3-ol (20.5 g, 48%). MS (ESI) m / z: 236.0 (M+H+).
[0158] 6-iodo-2-methylpyridin-3-ol (6.8g, 28.9mmol), 2,4-dichloropyridine (8.56g, 57.9mmol) and K2CO3 (4.00g, 28.9mmol) in DMA (50mL) The mixture i...
Embodiment 2
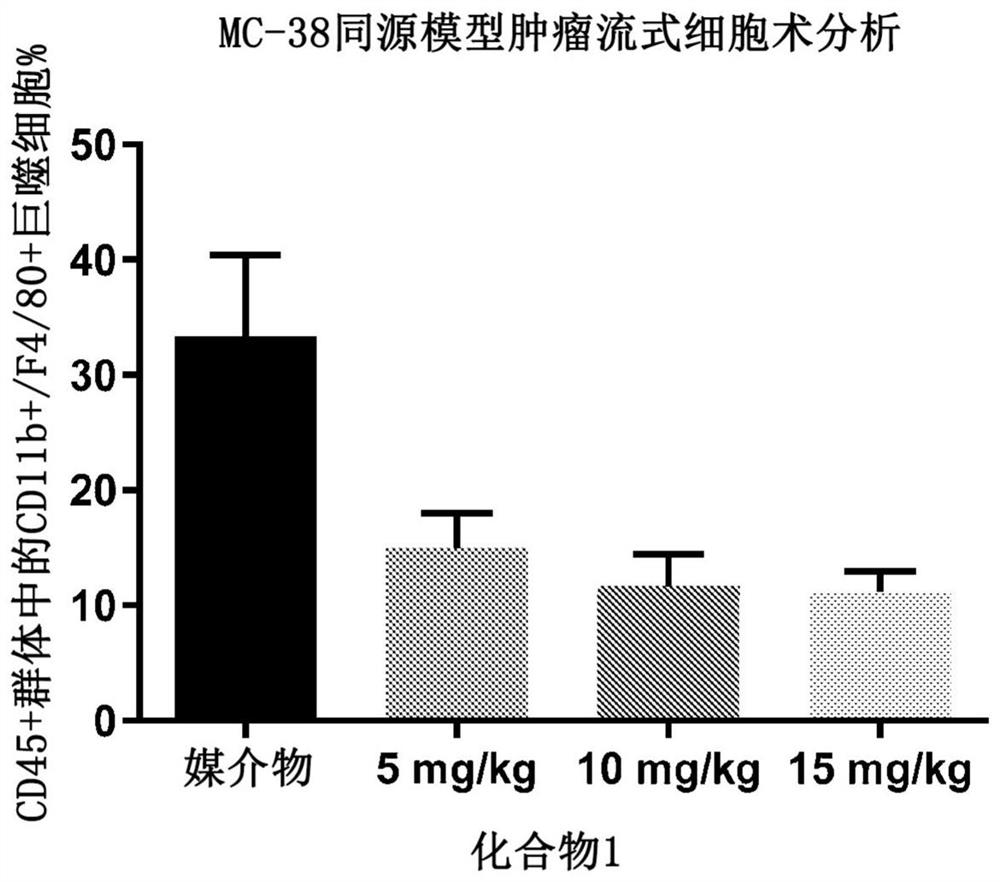
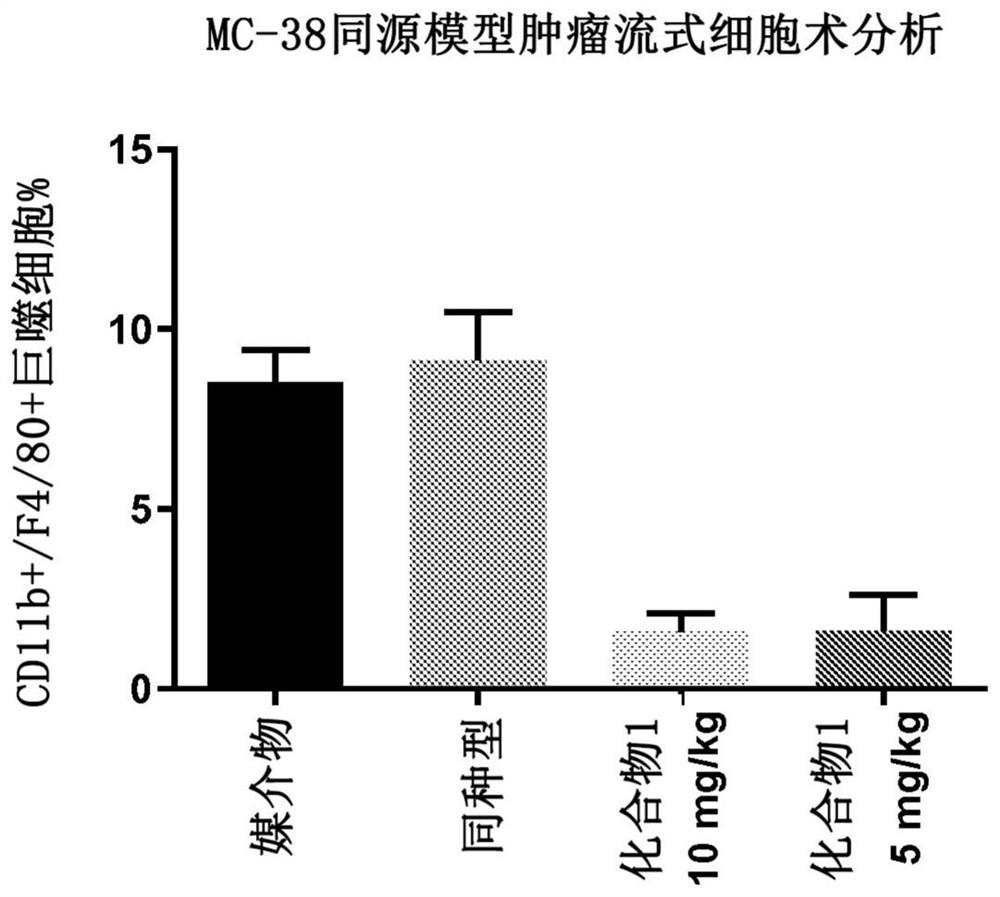
[0171] Example 2. Depletion of tumor-associated macrophages in a syngeneic mouse model of colorectal cancer
[0172]Protocols and procedures involving the following animal care and use of the syngeneic MC38 mouse xenograft model were reviewed and approved by the CrownBio Institutional Animal Care and Use Committee (IACUC) (Taicang, Jiangsu Province, China) prior to execution. During the study, animal care and use were performed according to the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All mice were given food and water ad libitum. All animals were observed for clinical signs at least once daily. In the first experiment, six to eight week old female C57BL / 6 mice were inoculated subcutaneously in the right lower flank with one million MC38 tumor cells in phosphate buffered saline. On day 12, when the tumor burden reached an average of 102 mm3, the mice were randomized. Groups of mice (n=10) were treated by oral gavage on days 12 to 18 a...
Embodiment 3
[0175] Example 3. Pharmacokinetic (PK) properties and depletion of circulating monocytes expressing CSF1R in treated patients
[0176] Doses of Compound 1 were administered orally and evaluated in seven dose cohorts totaling 40 patients with advanced solid tumor malignancies and TGCT. This included one dosing cohort receiving 10 mg QD; five dosing cohorts receiving a five-day loading dose regimen of up to 40 mg doses each, followed by a once-weekly or twice-weekly maintenance dosing schedule; and One cohort received a 5-day loading dose of 50 mg QD followed by 20 mg QD. Those five cohorts were: Cohort 1: 10 mg QD (n=7); Cohort 2: 5-day loading dose of 10 mg QD followed by a maintenance dose of 10 mg twice a week (twice a week or "BIW") ( 5x QD / BIW; n=3); Cohort 3: 20 mg QD 5-day loading dose followed by weekly 20 mg maintenance dose (5x QD / Q1W; n=4); Cohort 4: 20 mg QD 5-day loading dose , followed by a maintenance dose of 20 mg twice a week (5x QD / BIW; n=4); Cohort 5: 30 mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com