Cytisine orally disintegrating tablet and preparation method thereof
The technology of genistein and orally disintegrating tablets is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, medical preparations containing active ingredients, etc. The problem of low utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
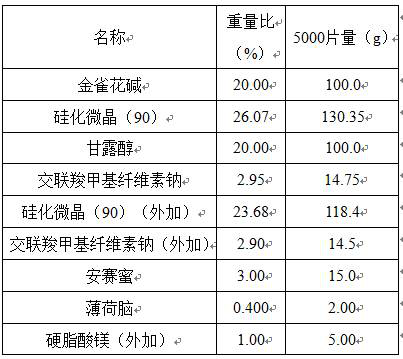
[0006] A kind of cytisine orally disintegrating tablet is made of components comprising the following percentages by weight:
[0007]
[0008] A method for preparing the above-mentioned cytisine orally disintegrating tablets by wet granulation, comprising the steps of:
[0009] Grind cytisine and pass through a 200 mesh sieve, add excipients in the prescription amount, 20% purified water as a wetting agent, granulate with a 40 mesh sieve, dry with air blast at 40°C, dry the moisture to 1~3%, pass the dried granules through Sieve with 30 meshes, weigh, convert yield, add siliconized microcrystal (90), menthol, acesulfame potassium and magnesium stearate, Φ6 flat punched tablets, tablet weight 150 mg, hardness 20-40 N.
Embodiment 2
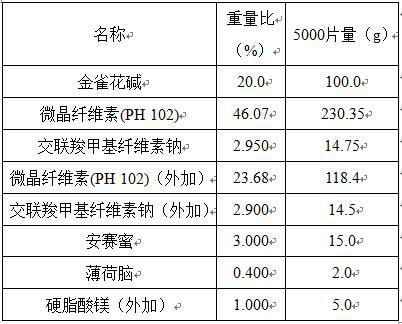
[0011] A kind of cytisine orally disintegrating tablet is made of components comprising the following percentages by weight:
[0012]
[0013] A method for preparing the above-mentioned cytisine orally disintegrating tablets by wet granulation, comprising the steps of:
[0014] Crush cytisine through a 200-mesh sieve, add excipients in the prescribed amount, 20% purified water as a wetting agent, granulate with a 30-mesh sieve, dry at 40°C with air blast, dry to 1-3% moisture, pass the dried granules through Sieve with 30 meshes, weigh, convert yield, add siliconized microcrystal (90), menthol, acesulfame potassium and magnesium stearate, Φ6 flat punched tablets, tablet weight 150 mg, hardness 20-40 N.
Embodiment 3
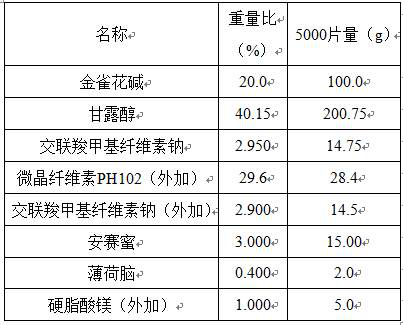
[0016] A kind of cytisine orally disintegrating tablet is made of components comprising the following percentages by weight:
[0017]
[0018] A method for preparing the above-mentioned cytisine orally disintegrating tablets by wet granulation, comprising the steps of:
[0019] Crush cytisine through a 200-mesh sieve, add excipients in the prescribed amount, 20% purified water as a wetting agent, granulate with a 30-mesh sieve, dry at 40°C with air blast, dry to 1-3% moisture, pass the dried granules through Sieve 30 meshes, weigh, convert yield, add silicified microcrystal (50), menthol, acesulfame potassium and magnesium stearate, Φ6 flat punched tablets, tablet weight 150 mg, hardness 20-40 N.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet weight | aaaaa | aaaaa |
| Sheet weight | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com