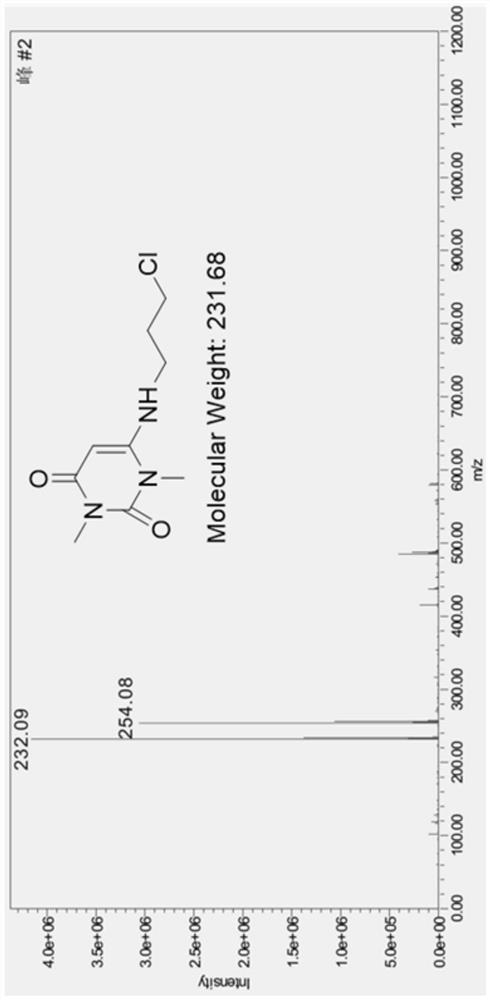
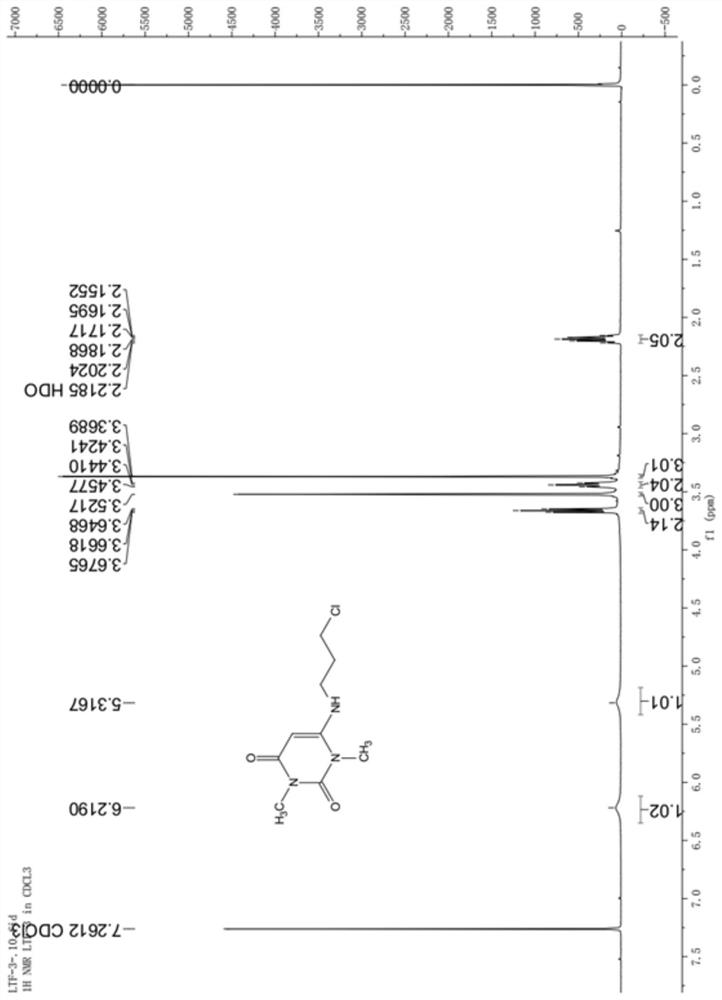
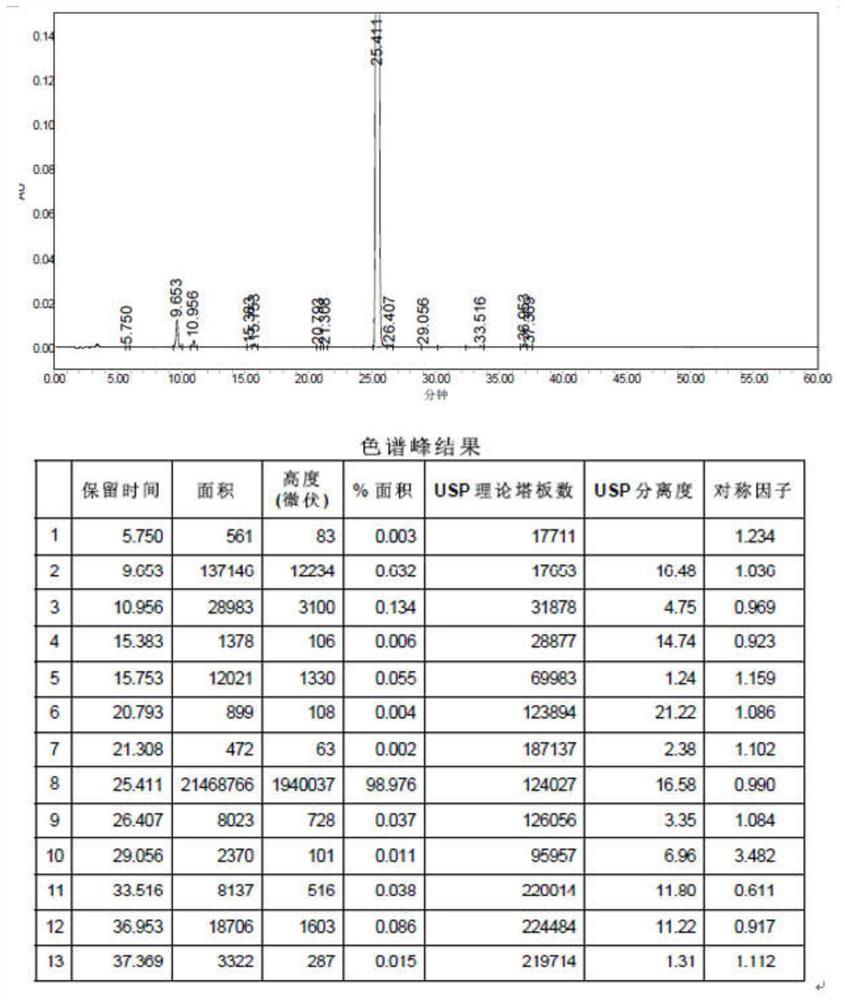
Preparation method of 6-(3-chloropropyl)amino-1,3-dimethyluracil
A technology of dimethyluracil and dimethyluracil, which is applied in the field of preparation of 6-amino-1,3-dimethyluracil, can solve the pressure of increasing the cost of labor protection and environmental protection, and increase the synthesis of drugs And drug quality analysis cost, human and environmental hazards and other issues, to achieve the effect of cheap raw materials, green and safe synthesis process, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Effects of different molar ratios of 6-(3-hydroxypropyl)amino-1,3-dimethyluracil II to thionyl chloride on product yield and purity:
[0022]
[0023] Carry out the reaction according to the above reaction equation, change the molar ratio of 6-(3-hydroxypropyl)amino-1,3-dimethyluracil II to thionyl chloride, fix the reaction temperature at 50°C, and the reaction time for 2 hours. After the end, the excess thionyl chloride was directly distilled off under reduced pressure at 45° C. (thionyl chloride can be recovered and reused). Then add 95% acetone pre-cooled to 5-15°C, cool down to below 0°C, keep stirring for 2 hours, and filter with suction. Rinse with 95% acetone and air-dry at 70°C for 10-12h.
[0024] The results are shown in Table 1:
[0025] Table 1
[0026]
[0027]
Embodiment 2
[0029] The preparation method is as in Example 1, the molar ratio of fixed 6-(3-hydroxypropyl)amino-1,3-dimethyluracil II to thionyl chloride is 1:3, the reaction time is 0.5 hours, and the post-treatment method As in Example 1, the impact of different reaction temperatures on product yield and purity.
[0030] The results are shown in Table 2:
[0031] Table 2
[0032]
Embodiment 3
[0034] The preparation method is as in Example 1, the molar ratio of immobilized 6-(3-hydroxypropyl)amino-1,3-dimethyluracil II to thionyl chloride is 1:3, the temperature is 50°C, and the post-treatment method As in Example 1, the impact of different reaction times on product yield and purity.
[0035] The results are shown in Table 3:
[0036] table 3
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com