Gaboxadol for reducing risk of suicide and rapid relief of depression
A gaboxadol, risk-based technology, applied in the field of gaboxadol for suicide risk reduction and rapid relief of depression, can solve problems such as failure every three days or more
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0198] Example 1: Whole Brain Drug Screening Platform
[0199] Numerous preclinical trials are currently being used to attempt to elucidate or predict the clinical effects of new drugs on the brain. These include in vitro high content screening (HCS) assays that measure the pharmacokinetics of drugs against specific molecular targets and their effects in simple cellular assays, at relatively low resolution (PET / CT, PET / MRI, fMRI) or Local responses at high cellular resolution (electrophysiological or two-photon imaging) to measure in vivo analysis of global responses, as well as to measure the behavior of animals performing in various tasks (Jain and Heutink, 2010; Judenhofer et al., 2008; Markou et al., 2009) analyze. Despite considerable effort in preclinical research, the clinical effects of drugs remain unpredictable, plaguing the drug development pipeline, resulting in clinical trial failure rates exceeding 90% (Pammolli et al., 2011).
[0200]The unique and novel appro...
Embodiment 2
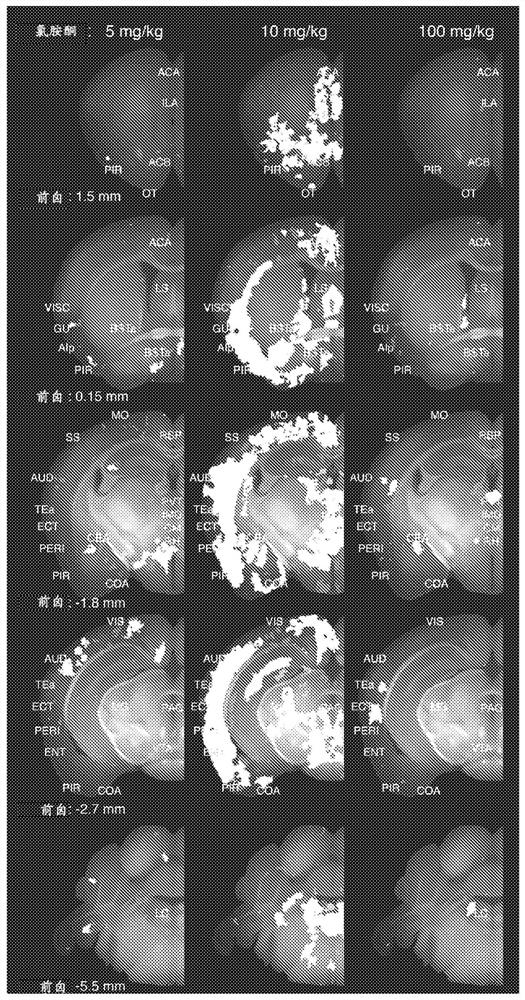
[0202] Example 2: Mapping of brain activation based on the effect of ketamine as a fast-acting antidepressant
[0203] Conventional antidepressants, when administered acutely in single doses, are chosen to match human equivalent doses used in clinical depression treatment to elicit discreet patterns of brain activation including frontal cortex, stria terminalis, bed nucleus ( BST), central amygdala (CEA), paraventricular hypothalamus (PVH), paraventricular thalamic nucleus (PVT) and locus coeruleus (LC) (Slattery et al, 2005; Sumner et al, 2004). Recently, intravenous ketamine, administered acutely at sub-anaesthetic doses, has been shown to act as a very rapid and robust antidepressant, producing a positive therapeutic effect within a few hours, rather than the typical two-fold required for the therapeutic effect of traditional antidepressants. to three weeks. While this exciting and novel clinical benefit of ketamine has been reproduced in numerous clinical studies, the mec...
Embodiment 3
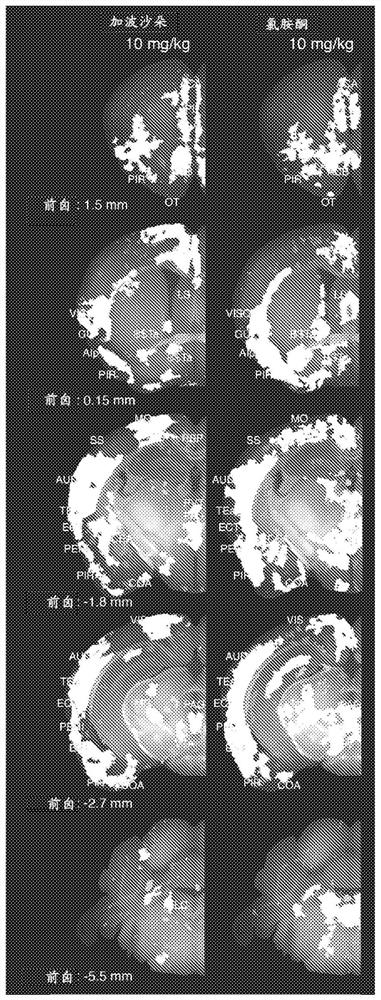
[0206] Example 3: Discovery of unexpected potential for gaboxadol to have rapid antidepressant and antisuicidal effects
[0207] Ketamine doses of 10 mg / kg elicited extensive activation in pharmacodynamic assays and were also shown to have acute effects in many behavioral studies in mice used to model depression, such as forced swimming, tail suspension, and learned helplessness. positive effects. Importantly, the corresponding HED of 50 mg ketamine per 60 kg male is in the human dose range of 0.5 to 1 mg / kg, which is used to achieve rapid antidepressant effects and reduce clinical depression even in treatment-resistant patients Suicidal ideation in patients. Therefore, our pharmacodynamic-based prediction is that the above-described 10 mg / kg ketamine-induced activation pattern represents a neuronal circuit-based mechanism of action for the rapid and significant therapeutic effects of ketamine in depression and suicidal ideation seen in the clinic. Based on this hypothesis, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com