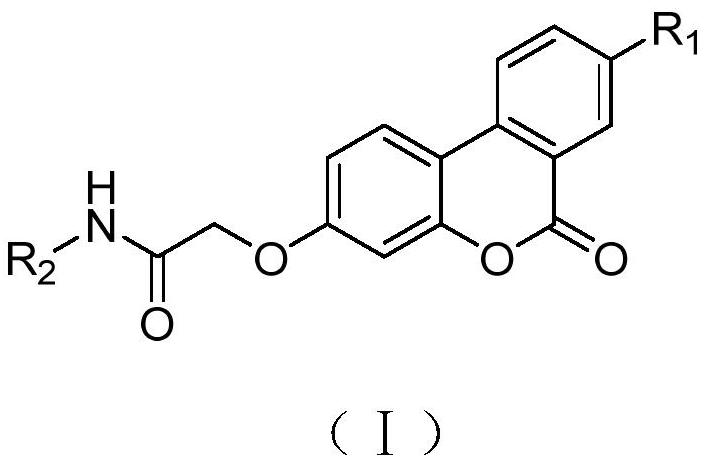
Urolithin PDE2 inhibitor compounds and preparation method thereof
A technology of PDE2 and urolithin, which is applied in the field of urolithin PDE2 inhibitor compounds and their preparation, and can solve problems such as no PDE2 inhibitors on the market
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
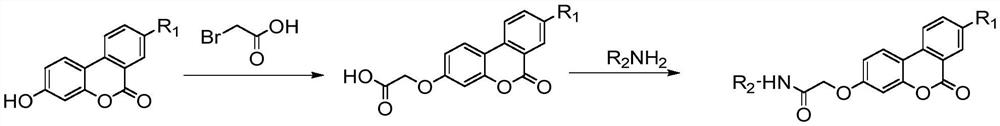
[0047] (1-1) 2-((6-oxo-6H-benzo[c]benzopyran-3-yl)oxy)acetic acid
[0048]
[0049] Add powdery white solid powder urolithin B (1mmol) and bromoacetic acid (5mmol), catalyst potassium carbonate (5mmol) and potassium iodide (1mmol) in a 500ml round bottom flask, add enough acetone solvent to make the compound dissolve. Condensate and reflux under the condition of 60°C in an oil bath, react for about 20 hours, and use TLC to detect whether the reaction is complete, wherein the developer is: methanol: dichloromethane = 1:5; The solution is clear and transparent, and the solution is light yellow. The pH value was adjusted to 3 with concentrated hydrochloric acid, and a light yellow solid was precipitated, filtered with suction, washed with a large amount of water, and dried to obtain the intermediate product 2-((6-oxo-6H-benzo[c]benzopyridine pyran-3-yl)oxy)acetic acid.
[0050] 2-((6-oxo-6H-benzo[c]benzopyran-3-yl)oxy)acetic acid (1-1): white solid, yield: 59.4%; M.p.>300°C; ...
Embodiment 2
[0055] (2-1) Synthesis of urolithin B amide derivatives (1a—1m)
[0056]
[0057] Dissolve the solid (1-1) obtained in the previous step in 20 mL of DMF at room temperature, add EDCl, HOBt, and DIEA in sequence and stir. After activating for 1 h, add dropwise R 2 NH 2 , stirred for a certain period of time, and followed the reaction with TLC. After the reaction, the reaction solution was added dropwise to saturated NaHCO 3 In the aqueous solution, a flocculent solid is formed, and after standing still, it is filtered, the solid is washed with a large amount of water, and dried to obtain the urolithin B amide derivative. Purify with methanol / dichloromethane at a volume ratio of 1:400.
[0058]
[0059]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com