Preparation formula of methacholine chloride freeze-dried powder for inhalation and preparation method of preparation formula
A methacholine chloride and freeze-drying technology, applied in the field of medicine, can solve problems such as not easy to absorb moisture and agglomerate, unsolved preparation production and sterility assurance, and high microbial level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
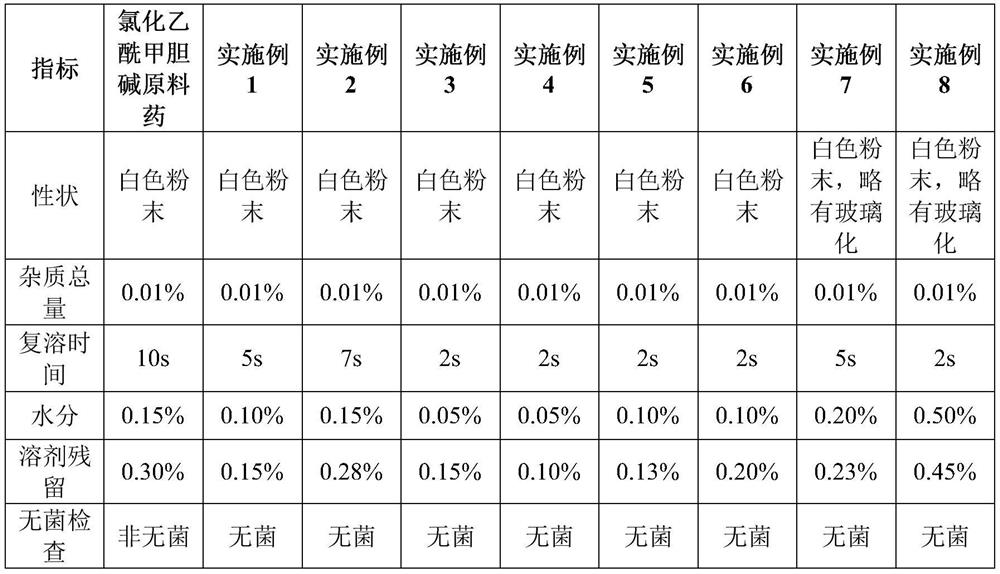
[0025] Embodiment 1, inhalation methacholine chloride powder
[0026] Weigh 1 g of homemade methacholine chloride crude drug, add 150 mL of ethanol, and stir until completely dissolved (the mass percentage of methacholine chloride is about 0.8%). The liquid medicine is sterilized and filtered through a 0.22 μm microporous membrane, filled in 20 mL vials, each bottle has a filling volume of 15 mL, half-filled with a rubber stopper, and placed in a lyophilizer for lyophilization. The medicinal solution is sterilized and filtered through a 0.22 μm microporous membrane, filled in vials, half-plugged with rubber stoppers, and placed in a freeze dryer for freeze-drying. The lyophilizer rapidly cools down to -40~-45°C and keeps warm for 120 minutes. After the cold trap cools down to -40~-45°C, start vacuuming, slowly raise the shelf temperature to -20~-30°C, keep warm for 9 hours, and slowly increase Shelf temperature to -10~10°C, keep warm for 4 hours, increase shelf temperature ag...
Embodiment 2
[0027] Embodiment 2, inhalation methacholine chloride powder
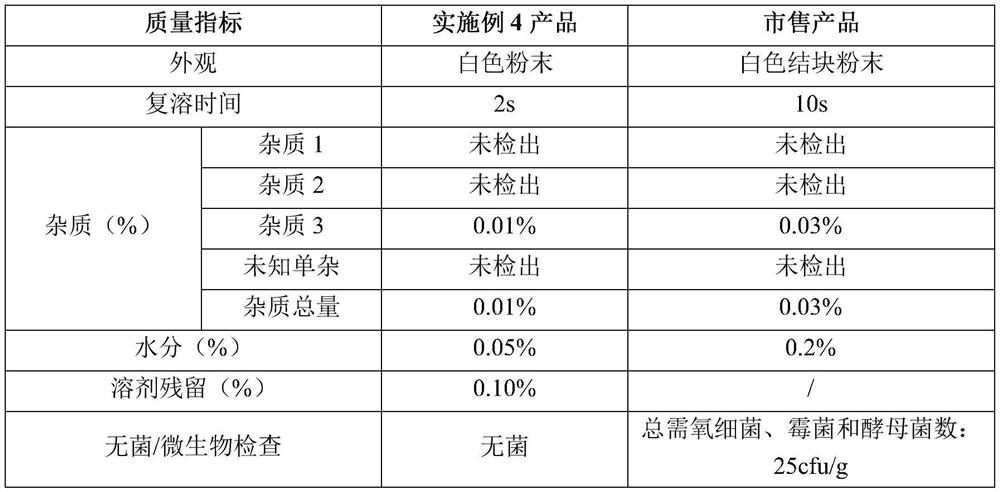
[0028] Weigh 1g of homemade methacholine chloride crude drug, add 1mL of water for injection, stir evenly, add propylene glycol to 50ml, stir until completely dissolved (mass percentage of methacholine chloride is about 2%). The medicinal solution is sterilized and filtered through a 0.22 μm microporous membrane, filled in 20 mL vials, each bottle filled with a volume of 5 mL, half-filled with a rubber stopper, and placed in a lyophilizer for lyophilization. The lyophilizer rapidly cools down to -40~-45°C and keeps warm for 240 minutes. After the cold trap cools down to -40~-45°C, start vacuuming, slowly raise the shelf temperature to -20~-30°C, keep warm for 9 hours, and slowly increase Shelf temperature to -10~10°C, keep warm for 3 hours, increase the shelf temperature again to 25~30°C, keep warm for 4 hours, keep the vacuum at 20~30Pa, and make the methacholine chloride product for inhalation; After the determi...
Embodiment 3
[0029] Embodiment 3, inhalation methacholine chloride powder
[0030] Weigh 1g of homemade methacholine chloride crude drug, add 0.2mL water for injection, stir evenly, add tert-butanol to 10ml, stir until completely dissolved (mass percentage of methacholine chloride is about 11.2%) . The medicinal solution was sterilized and filtered through a 0.1 μm microporous membrane, filled in 20 mL vials, each bottle filled with a volume of 1 mL, half-filled with a rubber stopper, and placed in a lyophilizer for lyophilization. The lyophilizer rapidly cools down to -45°C and keeps warm for 4 hours. After the cold trap cools down to -40~-45°C, start vacuuming, slowly (1 hour) raise the temperature of the shelf to -25°C, and keep warm for 9 hours; slowly (half an hour) ) raise the temperature of the shelf to -10°C and keep it warm for 1 hour; slowly (half an hour) raise the temperature of the shelf to 0°C and keep it warm for 1 hour; slowly (half an hour) raise the temperature of the sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com