Compounds for treating multiple myeloma
A multiple myeloma, compound technology, applied in the field of tenomustine, can solve the problem of toxicity and side effects of chemotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
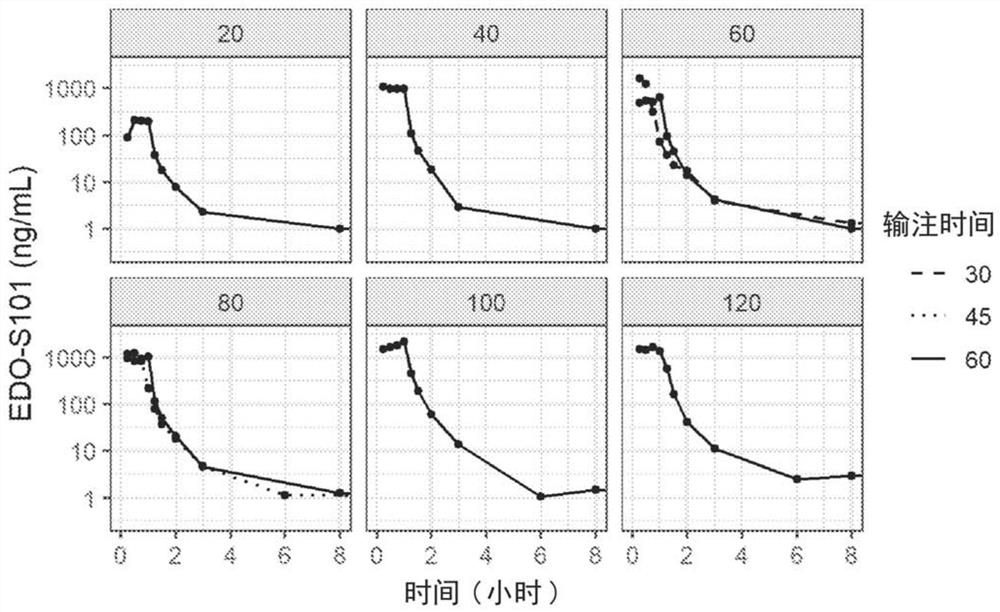
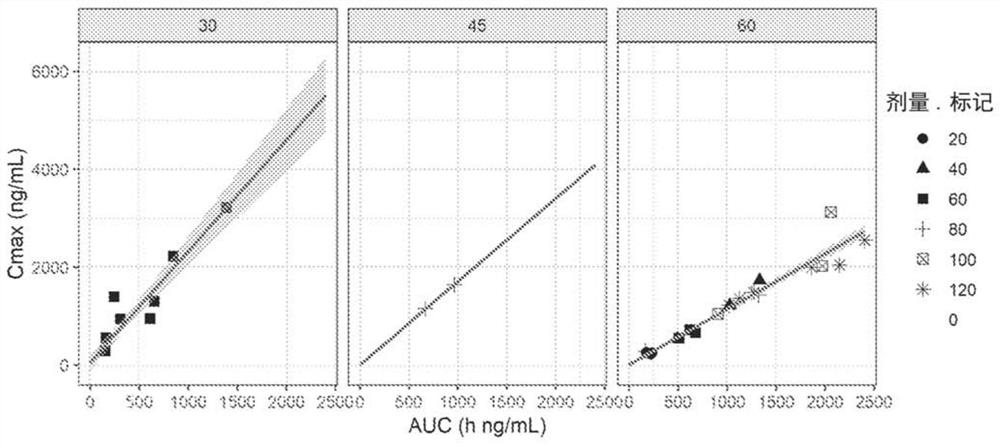
[0217] A Phase 1 trial including a dose escalation study was conducted to investigate the safety, pharmacokinetic (PK) profile of tenomustine (EDO-S101) in relapsed / refractory hematological malignancies Features and efficacy.
[0218] The patient had a relapsed / refractory hematologic malignancy for which there is currently no available therapy.
[0219] dose escalation level
[0220]
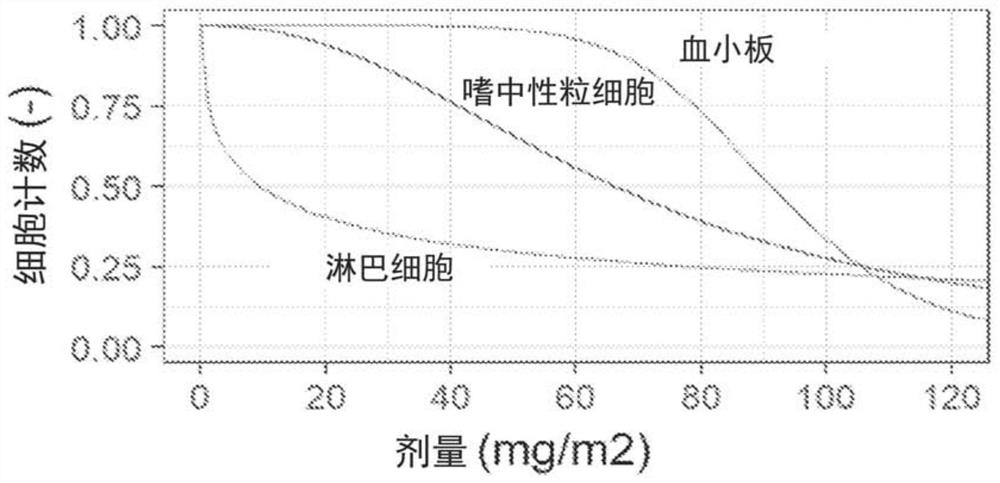
[0221] Dose-limiting toxicities (DLTs) were assessed based on Cycle 1 events only. Toxicity was assessed with respect to type and severity using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 (June 2010). Toxicity data were collected for all patients throughout their study period. Infusion site reactions were assessed using the Phlebitis Scale developed by the Association of Infusion Nurses (2011).
[0222] DLTs that are at least potentially related to study drug are defined as:
[0223] Any grade 3 or 4 non-hematological toxicit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com