Monoclonal antibody 20D8 of anti-SARS-CoV-2 epidemic mutant strain
A monoclonal antibody, sars-cov-2 technology, applied in antiviral immunoglobulins, instruments, peptides, etc., can solve the effect of monoclonal antibody neutralization activity, affect the transmission speed of mutant strains, pathogenicity, virus immunity Escape and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1, Preparation and Purification of Monoclonal Antibody 20D8
[0037] 1. Preparation: After injecting Freund's incomplete adjuvant 7 days in advance, monoclonal hybridoma cell lines (0.5mL, 3×10 6 cells / mL) were injected into the peritoneal cavity of mice, and cultured for 7-10 days.
[0038] 2. Purification: Harvest the ascites, let it stand at 37°C for 2 hours, centrifuge at 5000rpm for 30 minutes, collect the supernatant in the middle layer and filter it, and then purify it with a Protein G affinity chromatography column. The purification steps are briefly described as:
[0039] Equilibrate with 0.1M Tris buffer at pH 7.0;
[0040] After loading, rinse with 0.1M Tris buffer at pH 7.0;
[0041] Subsequent elution was performed with 1.0 M Tris buffer at pH 8.0.
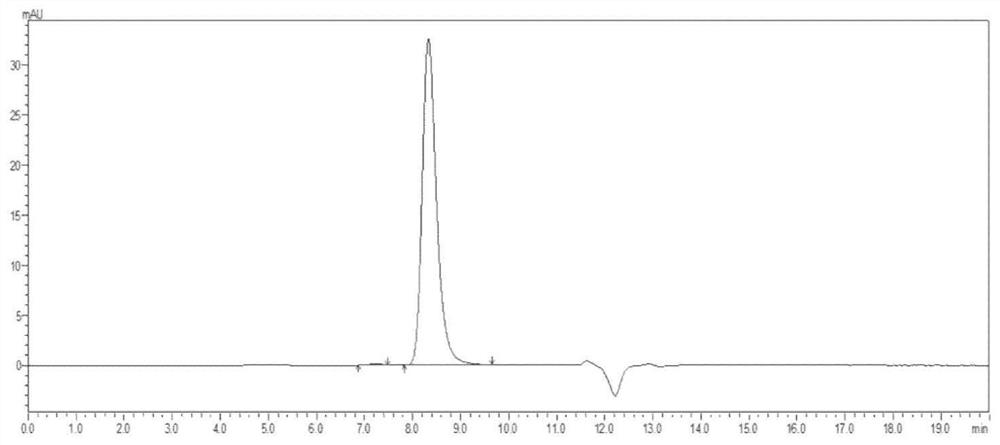
[0042] The eluate was collected and further dialyzed in PBS buffer. The purified antibody was taken for SDS-PAGE and HPLC-SEC detection and analysis.
[0043] 3. Result analysis: SDS-PAGE results ...
Embodiment 2
[0056] Example 2, Functional Analysis of Antibodies
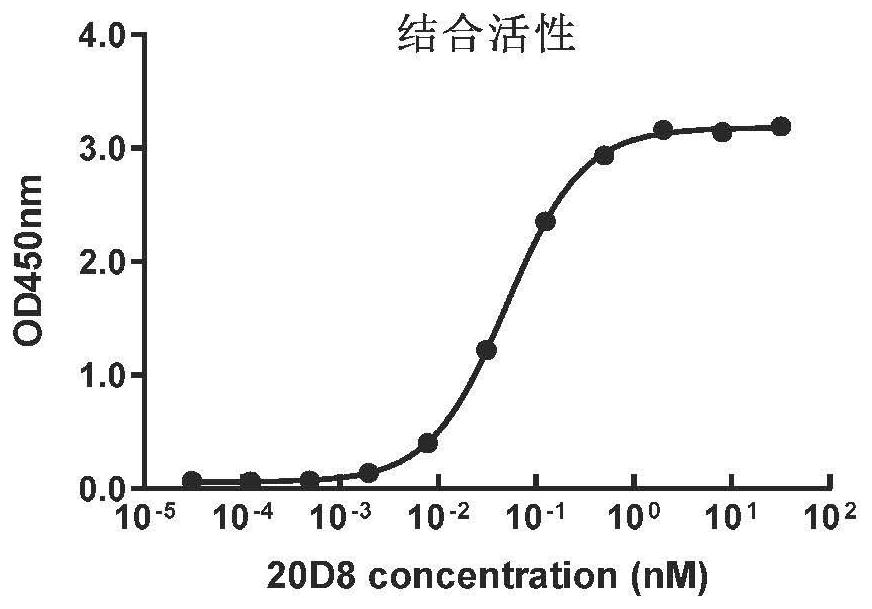
[0057] 1. Detection of binding activity of anti-SARS-CoV-2 monoclonal antibody 20D8 to antigen
[0058] The ELISA method was used to determine the binding ability of the 20D8 antibody to the spike protein S1 of the SARS-CoV-2 virus. The steps are briefly described as:
[0059] (1) Using SARS-CoV-2 spike S1-His recombinant protein (Sino company, product number: 40591-V08H) as the coating antigen, coat 2.0 μg / mL antigen on the microtiter plate with carbonate buffer, Incubate at 37°C for 2 hours;
[0060] (2) Block with casein buffer at 37°C for 2h; add serially diluted antibody to be tested, and incubate at 37°C for 1h;
[0061] (3) Add 1:10 000 diluted goat anti-mouse IgG-HRP (Biodragon Company, product number: BF03001X), and incubate at 37°C for 1 hour;
[0062] (4) After developing the color with the chromogenic solution, 2M HCl terminates the reaction; the absorbance A450nm value is detected by a microplate reader.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com