Chemotherapy-immunotherapy combined drug and preparation method and application thereof
A technology for chemo-immunity and chemotherapeutic drugs, applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve the problems of low level of tumor-infiltrating lymphocytes, low mutation rate of TNBC tumor cells, weak immune microenvironment, etc. Metastasis, inhibitory Treg reduction, growth inhibitory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0040] The synthesis of PEG-P(CL-DTC) block copolymer is based on MeO-PEG-OH ( M n = 2.0 kg / mol) is obtained by ring-opening polymerization of DTC and CL initiated by a macromolecular initiator and diphenyl phosphate (DPP) as a catalyst. Specifically, in an inert gas glove box, MeO-PEG-OH (800 mg, 0.4 mmol), DPP (1.0 g, 4 mmol), DTC (400 mg, 2.1 mmol), CL (400 mg, 3.5 mmol) and anhydrous DCM (3.2 mL), stirred and dissolved, sealed, removed from the glove box, and placed in a 40 ºC oil bath with magnetic stirring for 48 h. After the reaction was completed, glacial acetic acid was added to terminate the reaction, precipitated twice in 30 times the volume of glacial ether, filtered by suction, and dried in vacuum for 24 h to obtain PEG 2k -P(CL 1k -DTC 1k ), the preparation route is as follows:
[0041]
[0042] Using MeO-PEG-OH with a molecular weight of 5000 Da as the initiator and DPP as the catalyst, controllable ring-opening polymerization of CL and DTC according to ...
Embodiment 1
[0050] Example 1 Chemotherapy-immune combination drug
[0051] Preparation of PTX micelles
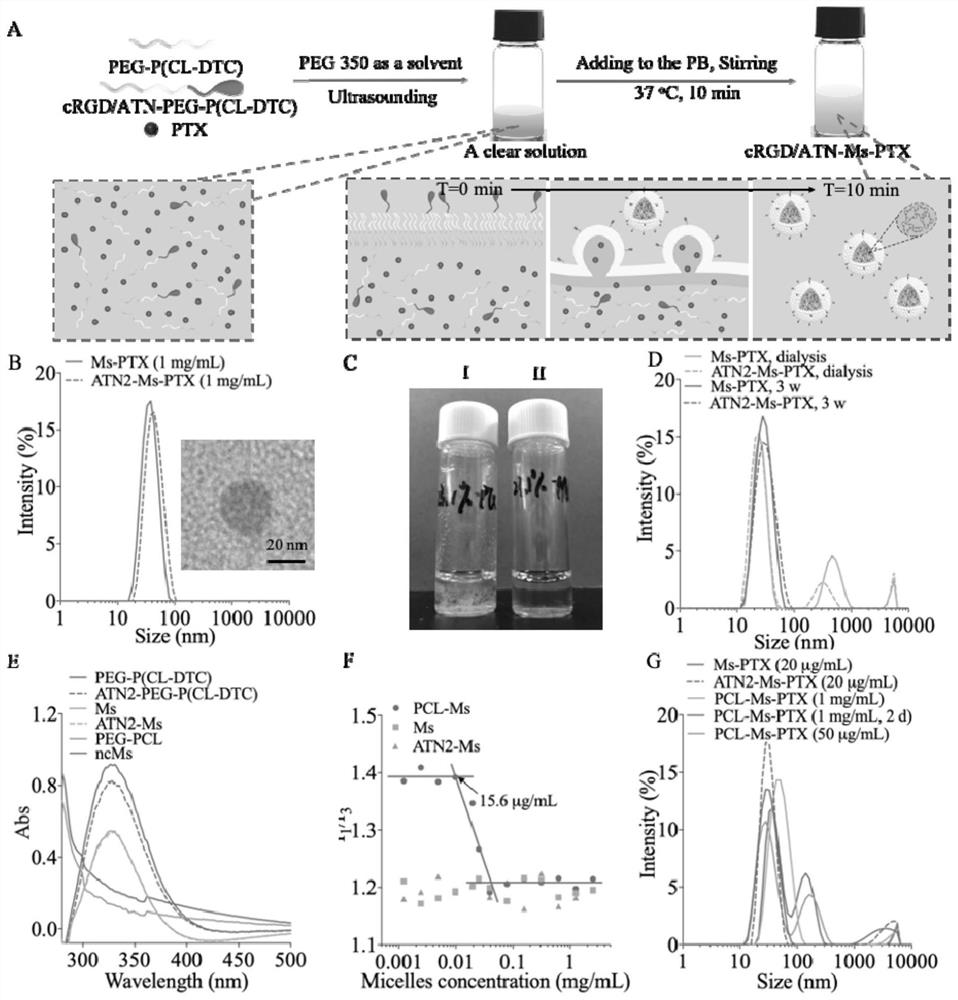
[0052] Combine PTX and PEG 2k -P(CL 1k -DTC 1k ) were dissolved in PEG 350 according to different mass ratios (5 / 100, 10 / 100, 20 / 100, 30 / 100), and the polymer concentration was 50 mg / mL. After conventional ultrasonication for 5 min, 100 mL of the mixture was poured into the bottom of 900 mL of PB solution (pH 7.4, 10 mM) at 37 °C, and stood without stirring during the process. After injecting, use a pipette gun to stick to the liquid surface and pipette 5 times to obtain a PTX drug loading of 4.8 wt. %~23.1 wt. % micellar MS-PTX, which is a non-targeting small micellar nanomedicine. The particle size and particle size distribution (PDI) were determined by DLS, and the micelle morphology was determined by TEM. MS-PTX (polymer concentration: 0.1 mg / mL) was dissolved in acetonitrile containing 20 mM DTT, and then the PTX concentration was tested by HPLC, and the drug loading and dr...
Embodiment 2
[0066] The targeting micelles loaded with PTX are composed of amphiphilic block polymer PEG 2k -P(CL 1k -DTC 1k ) and the polymer Ta-PEG-P (CL-DTC) coupled with targeting molecules (Ta is the polypeptide cRGD, ATN1 or ATN2) are self-assembled in the aqueous phase. 2.5%, 5%, 7.5%, 10% or 20% of ATN1-PEG-P (CL-DTC), ATN2-PEG-P (CL -DTC) or cRGD-PEG-P (CL-DTC) as the initial polymer solution, then mixed with the PTX solution, all the other steps are the same as in Example 1, to obtain micelles ATN1-MS-PTX, ATN2 with different polypeptide surface densities -MS-PTX and cRGD-MS-PTX, for targeting small micellar nanomedicine.
[0067] After mixing PEG-(CL-DTC) and targeting polymers coupled with cRGD, PHSCNK or PhScNK in different proportions, targeting micelles were prepared, and the same method as in Example 1 was used to keep the PTX drug loading at 4.8 wt. %, three series of micellar cRGD-MS-PTX, ATN1-MS-PTX and ATN2-MS-PTX with different targeting molecule densities were pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com